Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
By A Mystery Man Writer
Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
Bond length of H-H is 0-64 and the bind length of F2 is 1-2- Electronegativities of H and F respectively are 2-1 and 4-1-What is the bond length of HF- 1-0-64 2-0-92 3-0-82 4-0-62
Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62

WO2015057963A1 - Fgfr4 inhibitors - Google Patents
In which OO bond is length greater, H2O2 or F2O2? - Quora

Chapter 1-5 PDF, PDF, Neutron

Inorganic Chemistry For The JEE Mains and Advanced by K Rama Rao, PDF, Atoms

Phosphorescent organic light-emitting devices: Iridium based emitter materials – An overview - ScienceDirect
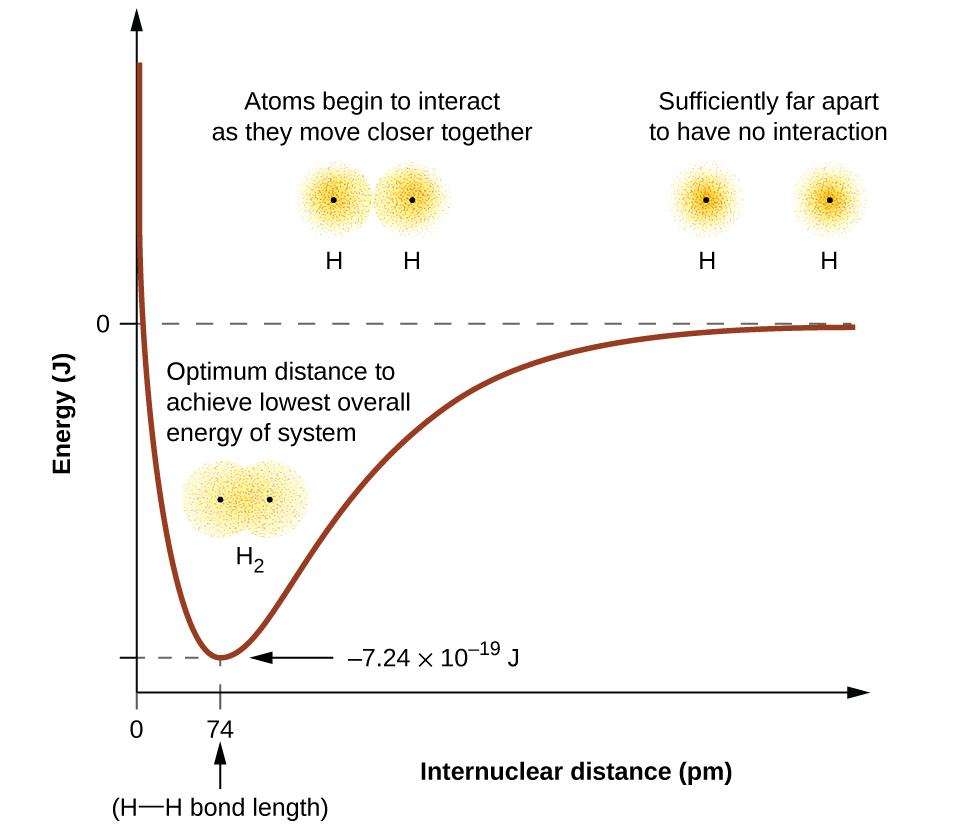
Valence Bond Theory – Atoms First / OpenStax

Write the increasing order of bond energies of H2,F2 and HF molecules.

Actinides - Chemistry and Phys. Properties - Structure & Bonding v.59 (1985) Pp.1-298, PDF, Electron Configuration

Assertion: The O O bond length in H2O2 is shorter than that of O2F2.Reason: H2O2 is an ionic compound.

Chapter 1-5 PDF, PDF, Neutron
What is the change in bond length of hetronuclear molecules due to difference of electronegativity value of bonded atom? - Quora

Chemical Engineering Reviewer Edited, PDF, Molecules
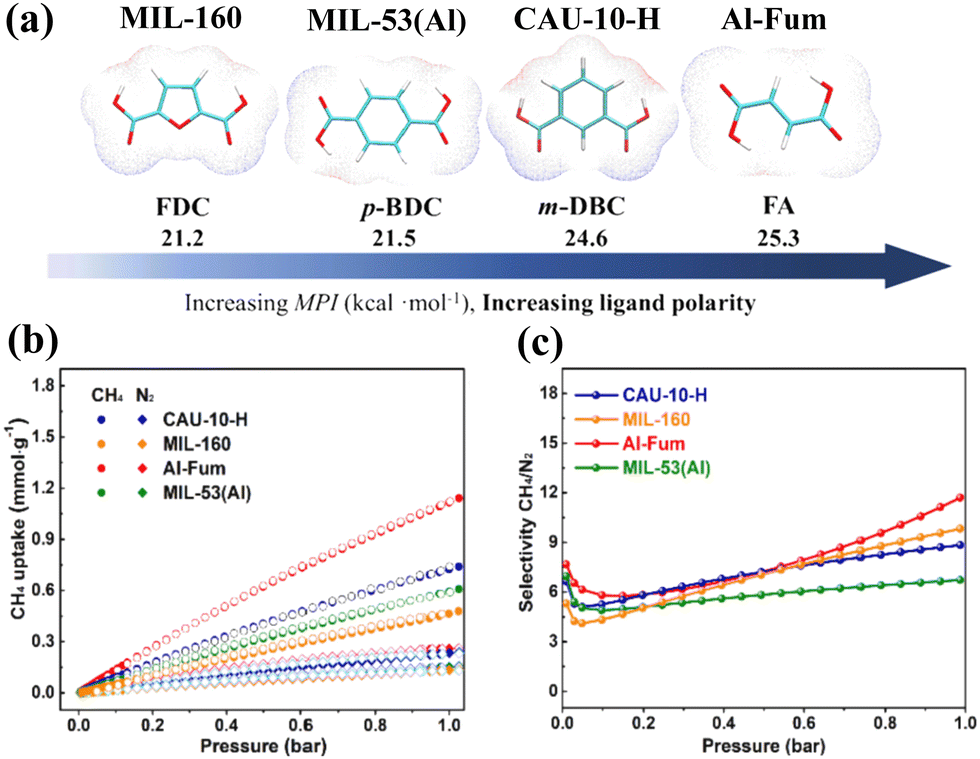
Non-CO 2 greenhouse gas separation using advanced porous materials - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D3CS00285C

Which of the following has largest bond angle? (a) H_2O (b) F_2O (c) Cl_2O (d) H_2 S
- Natural Looking Wholesale human micro braiding hair Of Many Types

- Intel Core i9-10900K Desktop Processor 10 Cores up to 5.3 GHz Unlocked LGA1200 (Intel 400 Series Chipset) 125W : : Electronics

- Lululemon Cross My Heart Bra Coal Gray Women's Size 6 - beyond

- Women Sexy Lace Lingerie Ultra-thin Transparent Bra Set Underwire Push Up Bras Black White Underwear Panty Sets Female Bralette - AliExpress

- Xssential Cropped Hoodie