Sacituzumab Earns Regular FDA Approval for TNBC - NCI

By A Mystery Man Writer
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.

PDF) Exploiting Therapeutic Vulnerabilities in Triple-Negative

Sacituzumab Govitecan - an overview

Mission Mountain Wilderness

Another Player in Advanced Bladder Cancer

Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer—phase 3 ASCENT study subanalysis

FDA Approves Sacituzumab Govitecan for Triple-Negative Breast
Sacituzumab Earns Regular FDA Approval For TNBC NCI
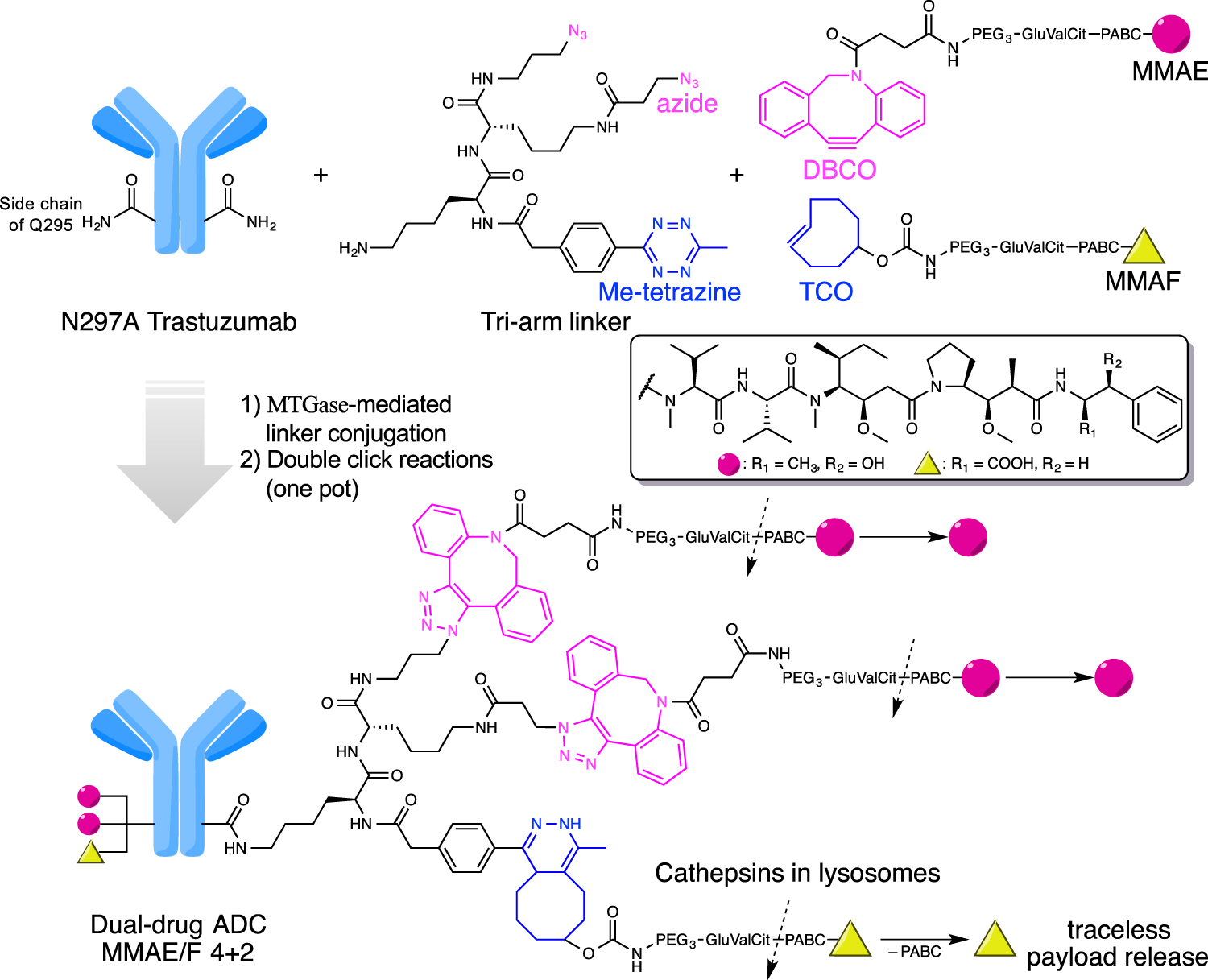
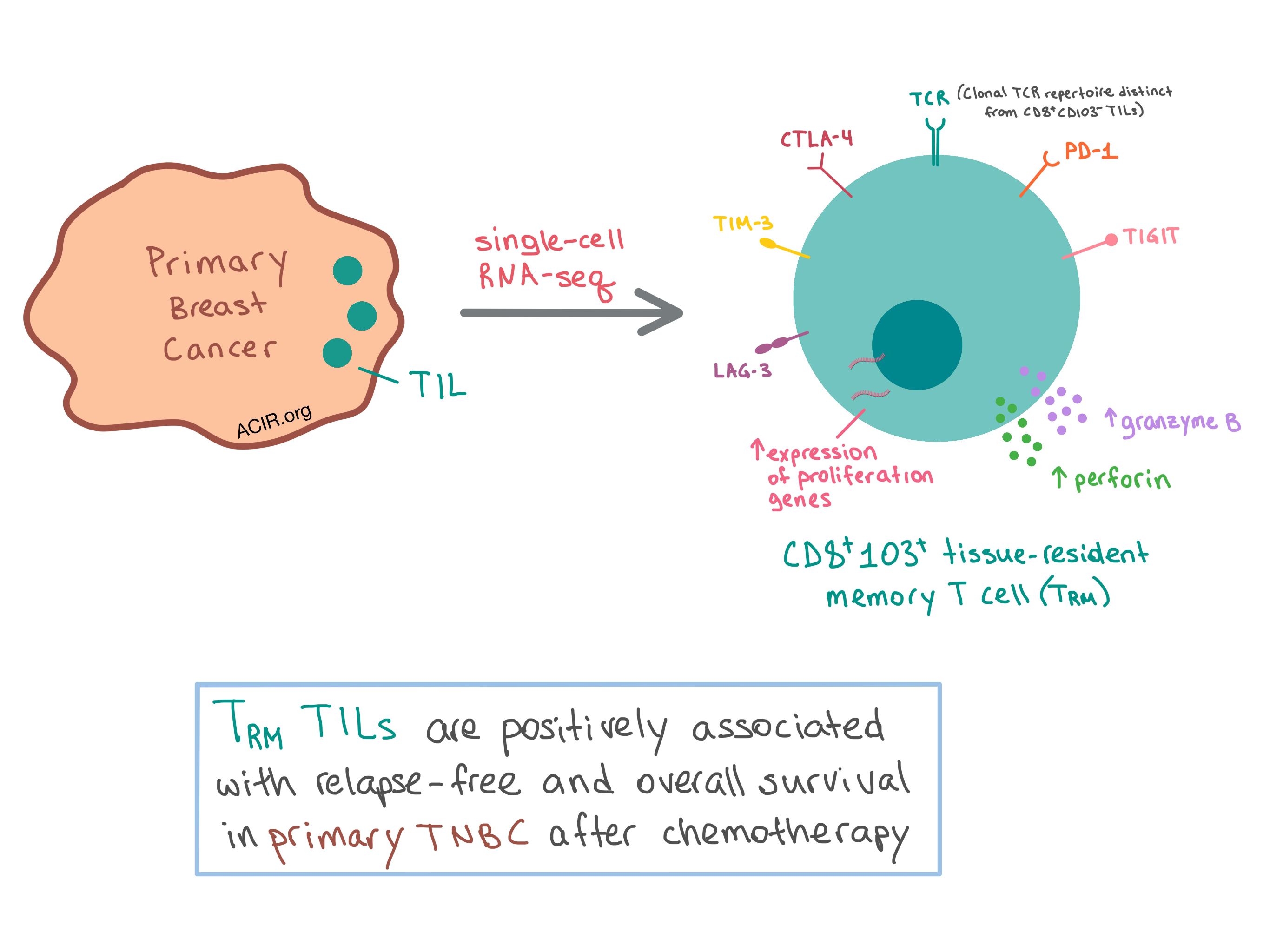
Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance

Targeting Triple-negative Breast Cancer

lyel-20221231
- Full Coverage Bra Shapewear Incorporated Hide Back Fat Bra Plus-Size

- Women Designer Swimsuit Crop Top Vest + Swim Shorts Trunks Boxers Set Tracksuit Patchwork Shark Camo Swimwear Bikini C61711 From Sweet_products, $16.3

- Óculos De Sol Quadros 2023 Desenhos Animados De Moda Quadrado Anti

- Totatuit Women's Tracksuit Set 2 Piece Velour Loungewear Set Long

- CEP Calf Sleeves 4.0
_copy.png)