117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum

gas laws - Compressible Factor - Chemistry Stack Exchange

b 26. The compressibility factor 1 mole of a van der Waal's gas Boyle temperature is 1+ VIV-yo) Find the value of x + y. tronarding the van property?

Real Gases Introductory Chemistry

Compressibility factor for H_2 behaving as real gas is : (1) 1 (2) (1-a/RTV) (3) (1+Pb/RT) (4) RT
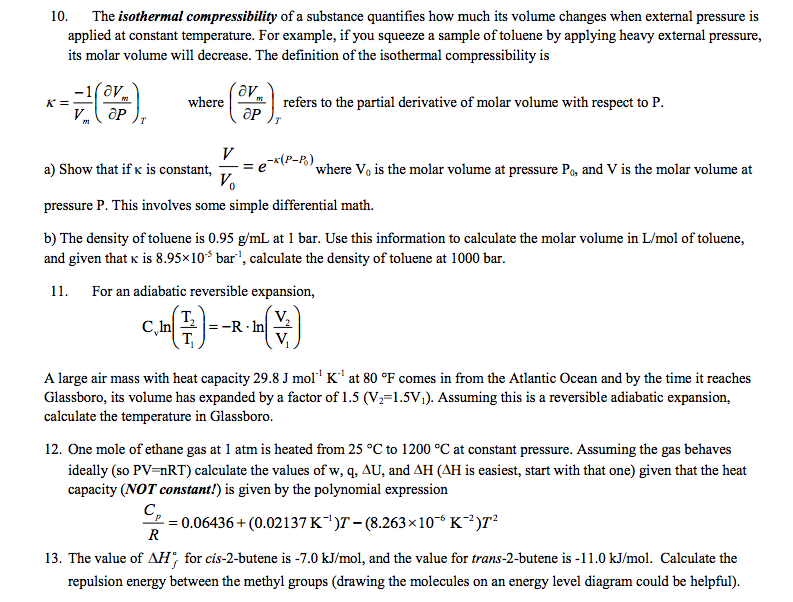
Solved 10. The isothermal compressibility of a substance

Magnesium based materials for hydrogen based energy storage: Past, present and future - ScienceDirect

ME2036- ENGINEERING THERMODYNAMICS BY Mr.P.SATHISH

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule

Solved We begin by showing that the compressibility factor

Solved Real gas effects can be expressed as departures from

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor (z): real gases deviate from ideal behav-Turito

Solved APPENDIX Problem 1: Molar Volume and Compressibility

Declaración XII Conf. Iberoam Mº Salud (español).tif - Segib
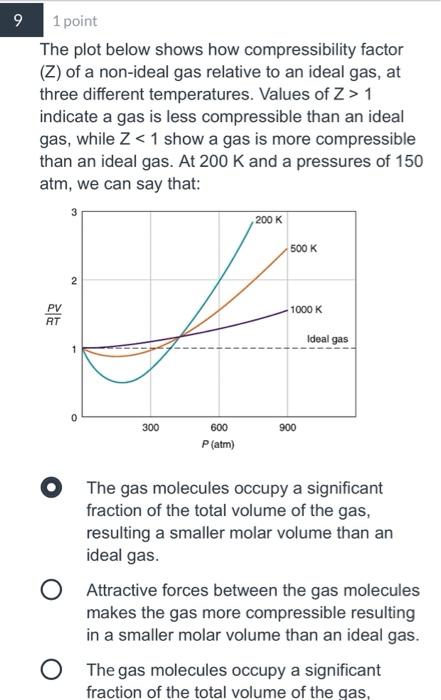
Solved 3 1 point Two gases, methane (CH4) and X, are
- Compressibility factor (Z) for a van der Waals real gas at critical point is

- Class Notes on Compressibility of a Real Gas, CH 417

- Which of the following statements is/are correct? (a) all real

- Developing a Thermodynamical Method for Prediction of Activity

- Non Ideal Gas Behavior-chemistry - Non Ideal Gas Behavior

- The Sophisticated! Invisible Beige Mid-Leg Faja Stagmi SMI7088 – Angelito's Boutique

- HSMQHJWE Womens Going Out Pants Women Casual Pants Suits Plus Size

- Well done Debbie Pyrah super slimmer & inspiration - Collette Walsh

- Exciting Journey of Y/n's First Pregnancy

- Useful Tips for Darker Colour on the Legs - Tansun
