How many moles of methane are required to produce 22 g of carbon dioxide? - Quora
By A Mystery Man Writer

Number of moles of methane required to produce 22g CO2(g) after combustion is x × 10–2 moles.

i.ytimg.com/vi/EjICJZdDcRo/hq720.jpg?sqp=-oaymwEhC
What is the number of moles in 54 kg water? - Quora
How to calculate the amount of carbon in 1 kg of sugar, C12H22O11 - Quora

How many moles of methane are required to produce 22 g CO2 (gram) after combustion?
How many moles of oxygen are required for the complete combustion of 75.0 g of ethane? - Quora
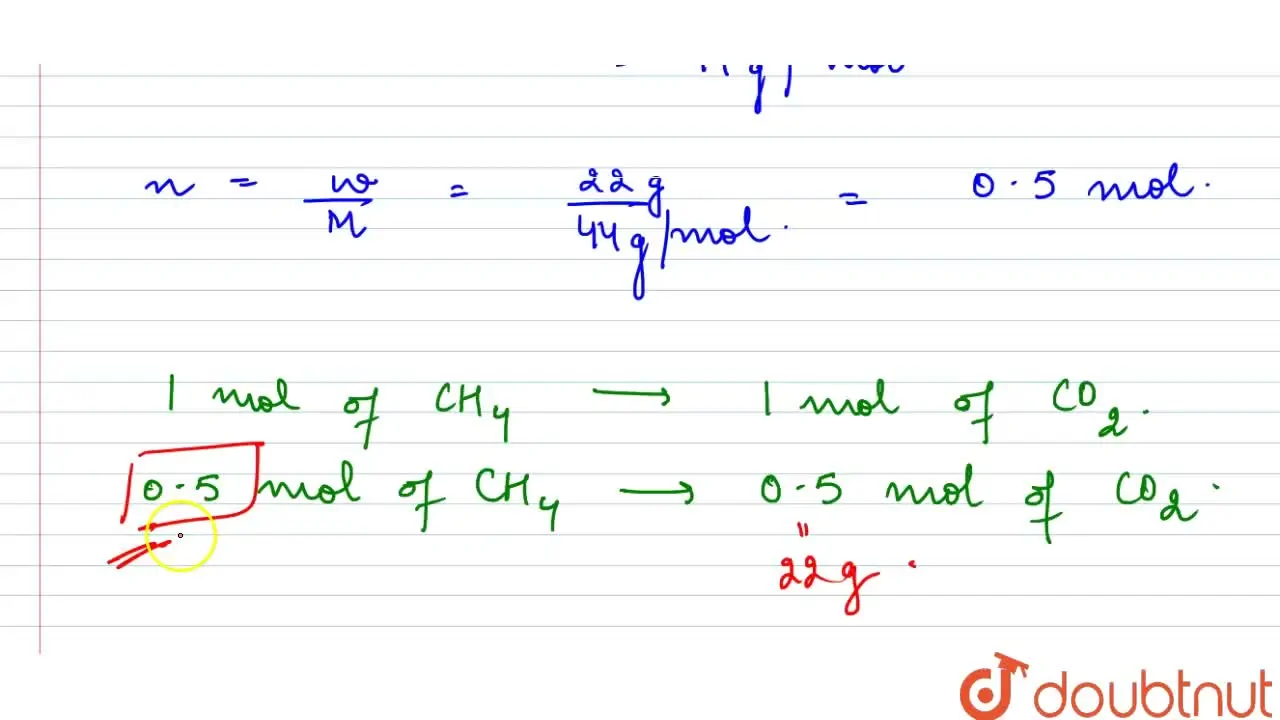
How many moles of methane are required to produce 22g of CO(2) on comb
How many carbon atoms are in 2.50g of butane? - Quora

(English) How many moles of methane required to produce 22g CO2 (g) after Combustion?
Iron is produced by the reduction of iron (III) oxide using carbon monoxide. Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g). How much Fe is produced from 1 kg of Fe2O3? - Quora
How to calculate the number of molecules of oxygen gas that occupies a volume of 224 ml at 273k and 3 atm - Quora

How many moles of methane are required to produce 22 g CO, (g) after combustion?
How many grams of oxygen are required to burn completely 570 grams Octane? - Quora
- Iluminador Pó de Fada 44 g - Vivai - Iluminador Facial - Magazine Luiza

- Fralda Descartável Infantil Cremer Magic Care G Pacote 44 Unidades - Supernosso

- BARRA METATARSAL COM PROLONGAMENTO INFRA CAPITAL 41/44 G
:quality(80)/anatofee/catalog/7683-4144-g.jpg)
- What mass of carbon is present in 44g of carbon dioxide? - Quora
- Bolachas Recheadas com Nutella 2 Unidades embalagem 44 g · Nutella





