Modular Medical submits next-gen insulin pump for FDA clearance

By A Mystery Man Writer
Modular Medical (Nasdaq:MODD) announced today that it submitted its next-generation MODD1 insulin pump to the FDA for 510(k) clearance.

as-ex99_1page019.jpg

Erica Scott - CSAM on LinkedIn: Modular Medical submits next-gen insulin pump for FDA clearance

10 updates about diabetes technology
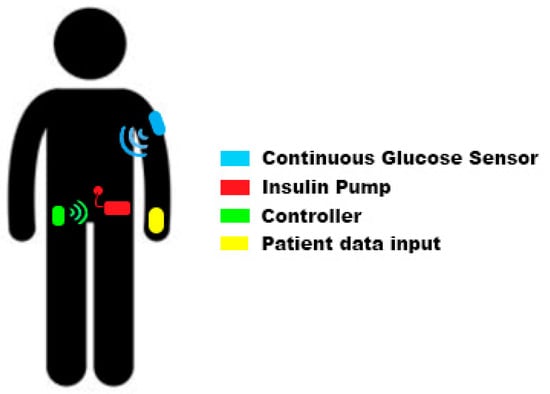
Electronics, Free Full-Text

In the News.. weight loss & cancer study for T2D, new pump submitted, Summer Olympic hopeful with T1D and more! - Diabetes Connections

Erica Scott - CSAM on LinkedIn: Medtronic nixes $738M deal for insulin patch pump maker EOFlow

Featured The Savvy Diabetic
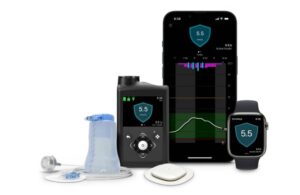
These diabetes devices are set to launch in 2024

A narrative commentary about interoperability in medical devices and data used in diabetes therapy from an academic EU/UK/US perspective

David Kliff on LinkedIn: Modular Medical submits next-gen insulin pump for FDA clearance
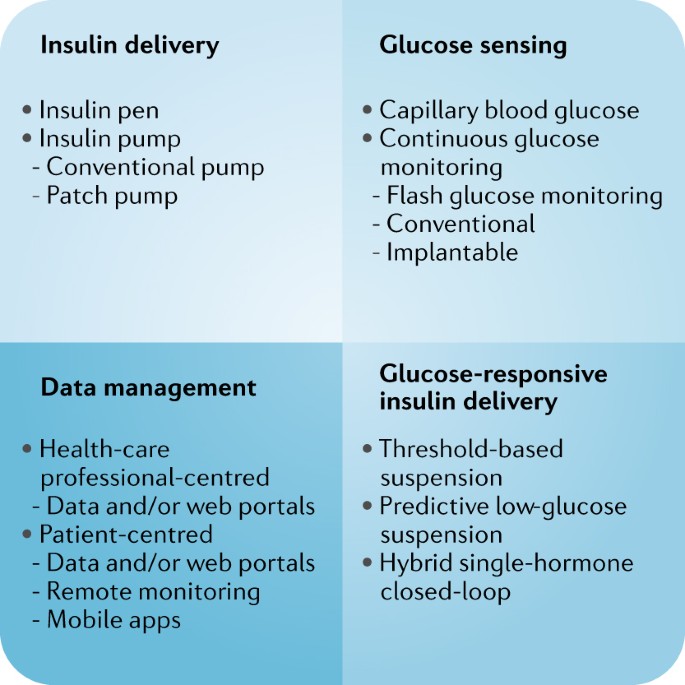
Technology in the management of type 1 diabetes mellitus — current status and future prospects

World's smallest insulin pump granted FDA clearance - Medical Device Network
- Smart Fit se prepara para abrir academias em mais três países

- Discover ALPHA 3.0 JACKET, Darkest Spruce for Your Adventures

- Buy Beurer Abdominal Toning Belt online in India. Best prices
- PINK Victoria's Secret Black Push up Bra Size 36B

- PEASKJP Shapewear Underwear Tummy Control Body Shaper Tank Tops Butt Lift Underwear, White L