SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.

By A Mystery Man Writer
VIDEO ANSWER: We can say permanganate permanganate iron, which is here, or we can say intense, intense purple colorati. The oxidation state of the manganese can be found in the m n, o 4 negative. Here, we can say that it is plus 7. The electrons are
[MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2. d-d transitions in the Mn compound compared to the Re compound 3. charge transfer from O to Re compared to O to Mn 4. charge transfer from O to Mn compared to O to Re.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

The purple color of KMnO4 is due to:

Solution of MnO(4)^(-) is purple-coloured due to

Assertion: The purple colour of KMnO4 is due to the charge transfer tr

SOLVED: [MnO4]- is deep purple in color whereas [ReO4]- is colorless. This is due to greater energy required for 1. d-d transitions in the Re compound compared to the Mn compound 2.
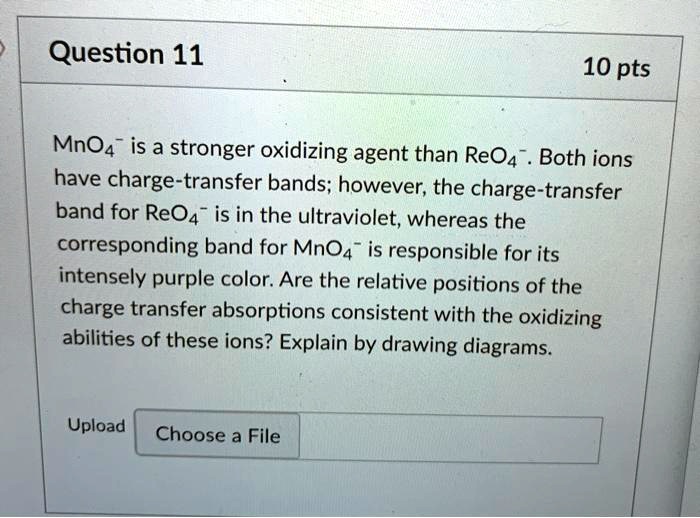
SOLVED: MnO4 is a stronger oxidizing agent than ReO4. Both ions have charge-transfer bands; however, the charge-transfer band for ReO4 is in the ultraviolet, whereas the corresponding band for MnO4 is responsible

The color of KMnO4 is due to

4. Solution of Mno, is purple-coloured due to (A) d-d-transition (o due to both d-d-transition and charge transfer (B) charge transfer from O to Mn (D) none of these The ionisation enemies

Solved The ion MnO4 has Mn in the +7 oxidation state with no
Why does KMnO4 show colour instead of no unpaired in Mn+7 (4s° 3D°)? - Quora

Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by S.Dharmaraj - Issuu

Solved Part 1 - Variety of Permanganate Redox Reactions The

Assertion: The purple colour of KMnO4 is due to the charge transfer tr
- Patch Plush in Swaddle – 101 Dalmatians – Disney Babies – Small 10
- Women's Tops Clearance
- Sleeping Beauty Cosplay Wig Aurora Princess Elora Long Curly Wavy Hair Wigs Halloween Fancy Dress Brown Anime Wig Costume Wig For Adult Women Party New Synthetic Hairpiece : : Beauty & Personal

- Faja Colombiana con Cremallera Reduce 2 Tallas al Instante – Fajas Latinas

- Brownmed IMAK RSI Pil-O-Splint