The value of compression factor at the critical state of a vander waals gas is
By A Mystery Man Writer
The value of compression factor at the critical state of a vander waals gas is

The compressibility factor of H(2)(g) at its critical condition if it

thermodynamics - Negative Pressures in Van der Waals Equation of
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
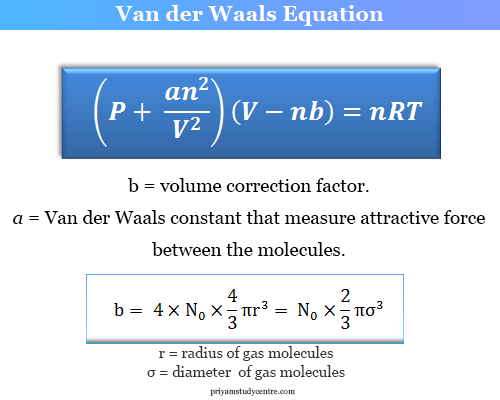
Van der Waals Equation - Derivation, Formula, Units - Chemistry
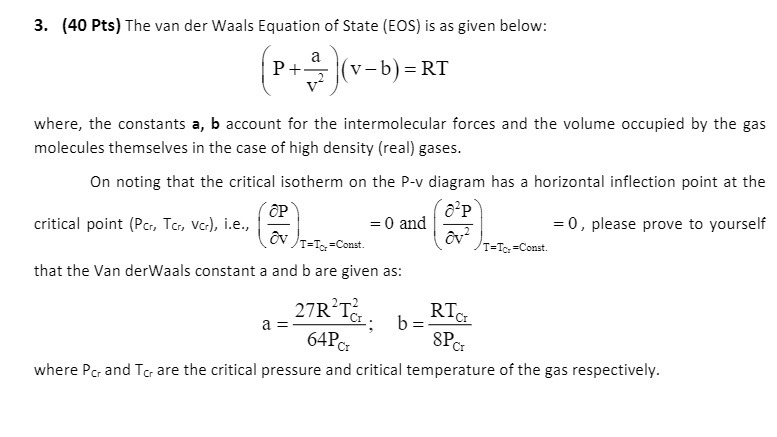
SOLVED: Please prove to yourself that the Van der Waals constants a and b are given as: 3. (40 Pts) The van der Waals Equation of State (EOS) is as given below: (

At high pressure, the compressibility factor for one mole of van der w

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
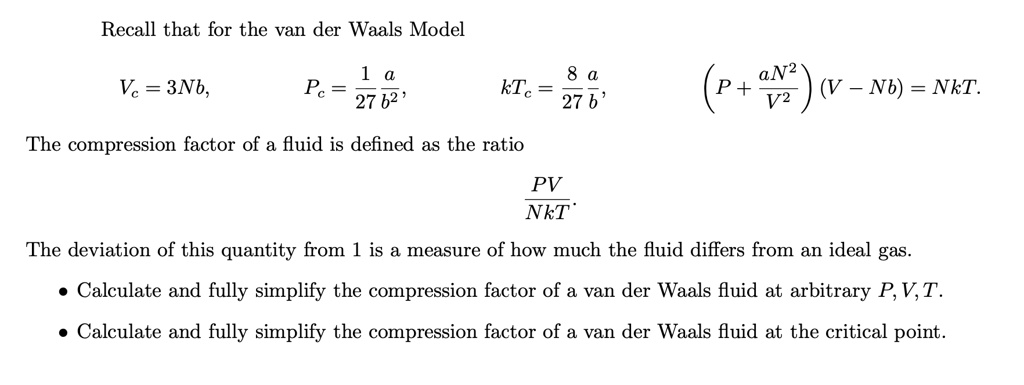
SOLVED: Recall that for the van der Waals Model: Vc = 3Nb, Pc = 27b^2, kTc = 27b, aN^2P + (V - Nb) = NkT/V^2 The compression factor of a fluid is
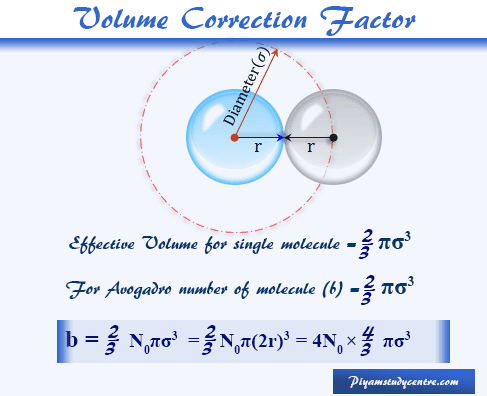
Van der Waals Equation - Derivation, Formula, Units - Chemistry
- Excel Calculations: Compressibility Factor for Natural Gas
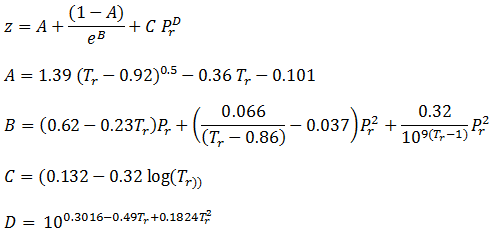
- Compressor and jet vacuum system:, by Maryambotshekan

- The compressibility factor is Z = PV/R_g T. Evaluate

- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
- At Critical Temperature,pressure and volume . The compressibility




