In the following compressibility factor Z vs pressure graph at 300

By A Mystery Man Writer
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state
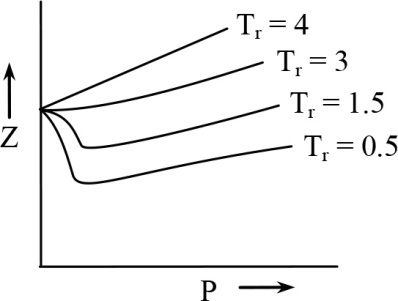
Which of the following plots isare correct Tr is reduced temperature

2nd Year Holiday Assignment, PDF, Tangent
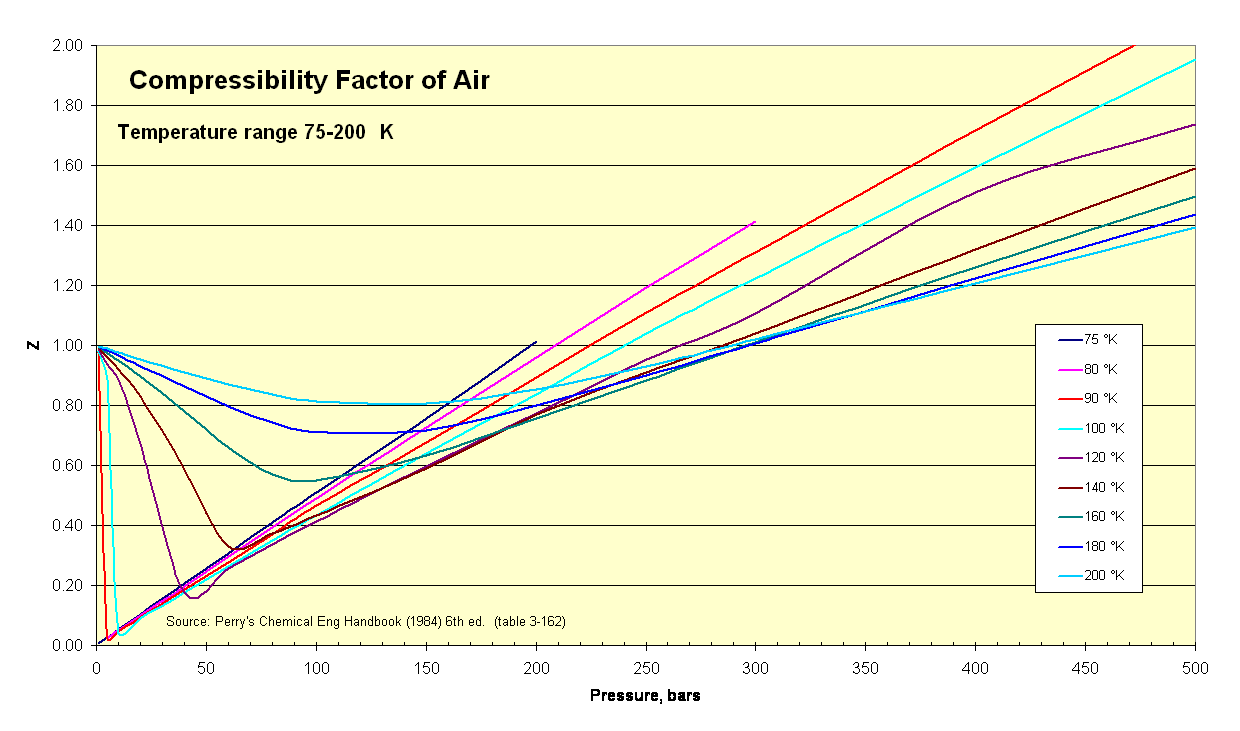
Air Compressibility Factor Table - EnggCyclopedia

Compressibility factor (Z) is plotted against pressure at different te

Kvpy 4, PDF, Electronvolt

Compressibility factor - Wikipedia
Real-world gas calculations

2nd Year Holiday Assignment, PDF, Tangent

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
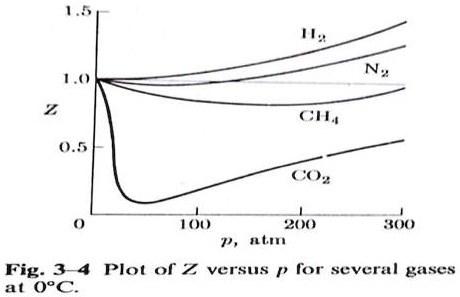
SOLVED: Subject: Compressibility Factor What is the analysis of the graph? 1.5 N 1.0 Z CHA 0.5 CO2 1 0 100 200 P, atm 300 Fig. 3-4: Plot of Z versus p for several gases at oc.

47. In the following compressibility factor (Z) vs pressure graph 300 K, the compressibility factor of CH4 pressures < 200 bar deviates from ideal behavior because

What is the expression of first law of thermodynamics for adiabatic process

Compressibility factor (gases) - Citizendium
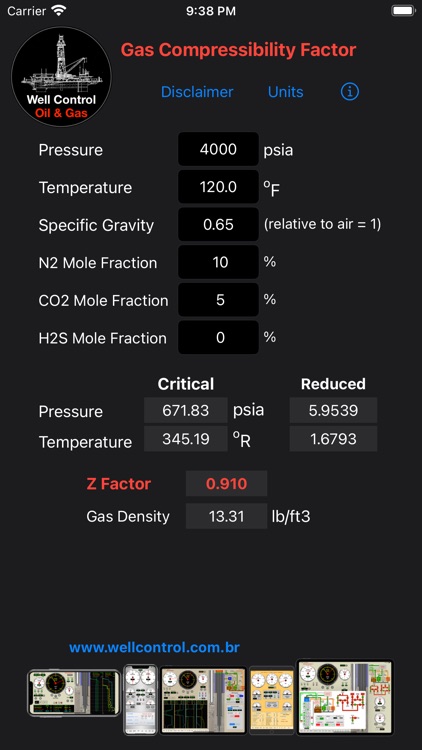
- Gas Compressibility Factor Z by Carlos Moura
- For $CO$, isotherm is of the type as shown. Near the point

- Solved The plot below shows how compressibility factor (Z)
- Write the expression for the compressibility factor (Z) for one

- New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

- Pack of 2 pairs of women’s second skin ankle socks in black

- Rupa Boys Thermal Set

- Transparent PVC Underpants Adult Sexy Panties Incontinence Shorts Plastic Pants Clear Nappies Glass abdl

- GOYARD SAINT SULPICE RED CARD HOLDER

- 10Pcs 5Pairs Mop Slippers for Floor Cleaning,Microfiber Mop Slippers Shoes Mop Socks Floor Cleaning Tools Foot Shoe Cover Soft Washable Reusable

