At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

By A Mystery Man Writer
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

Q.6 At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given RT as p = V-b T.

Solved In general, real gases behave most ideally at

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior.

PDF) Thermal energy storage Diego Armando Gutierrez Diaz

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior.

The temperature of an ideal gas is increased from 27∘ C to 127∘ C. Then, percentage increase in V rms isA. 37 %B. 11 %C. 33 %D. 15.5 %

GK Theory + GK Quiz & Business Quiz + Business Compendium 1 - 202 Pages

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..
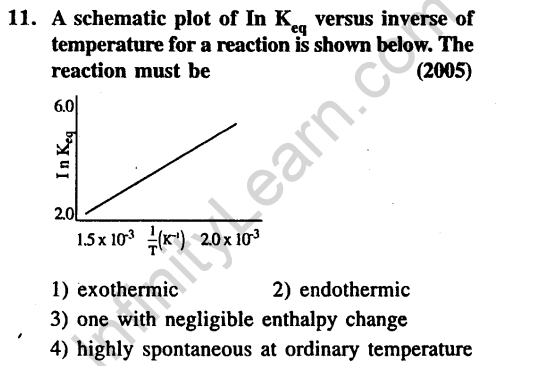
Thermodynamics and Chemical Energitics- JEE Main MCQ'S & Solutions
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- Answered: Compression factor of a gas with van…

- Derive an expression for the compression factor of a gas tha
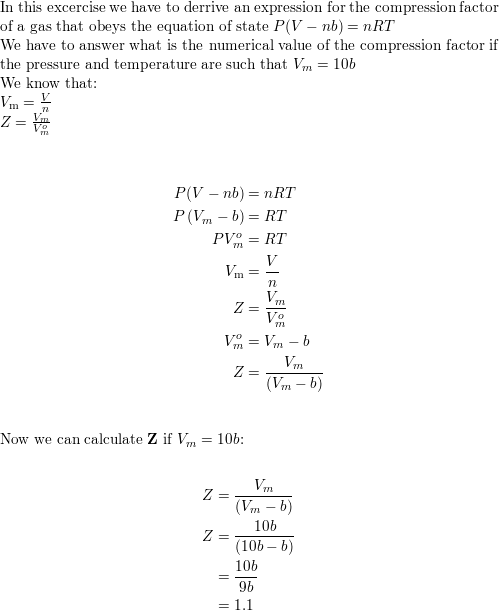
- At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

- What is the value of compression factor Z for the gas? (A) 1 (B) >1 (C) <1 (D) Zero





