Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

By A Mystery Man Writer

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
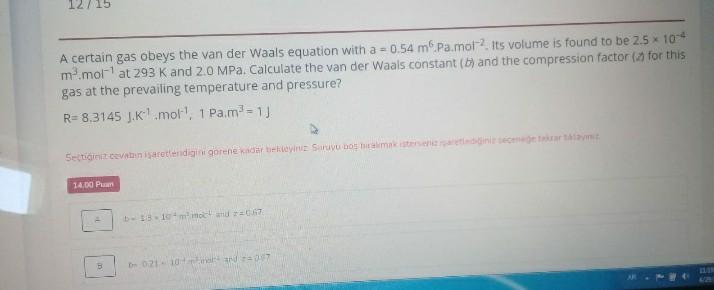
Solved A certain gas obeys the van der Waals equation with

At high pressure, the compressibility factor for one mole of van der w

Graeff's experiments and 2LoD: Replication and Implications
The compression factor (compressibility factor) for one mole of a van der Waals' gas at 0ºC and 100 atm pressure is - Sarthaks eConnect

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}
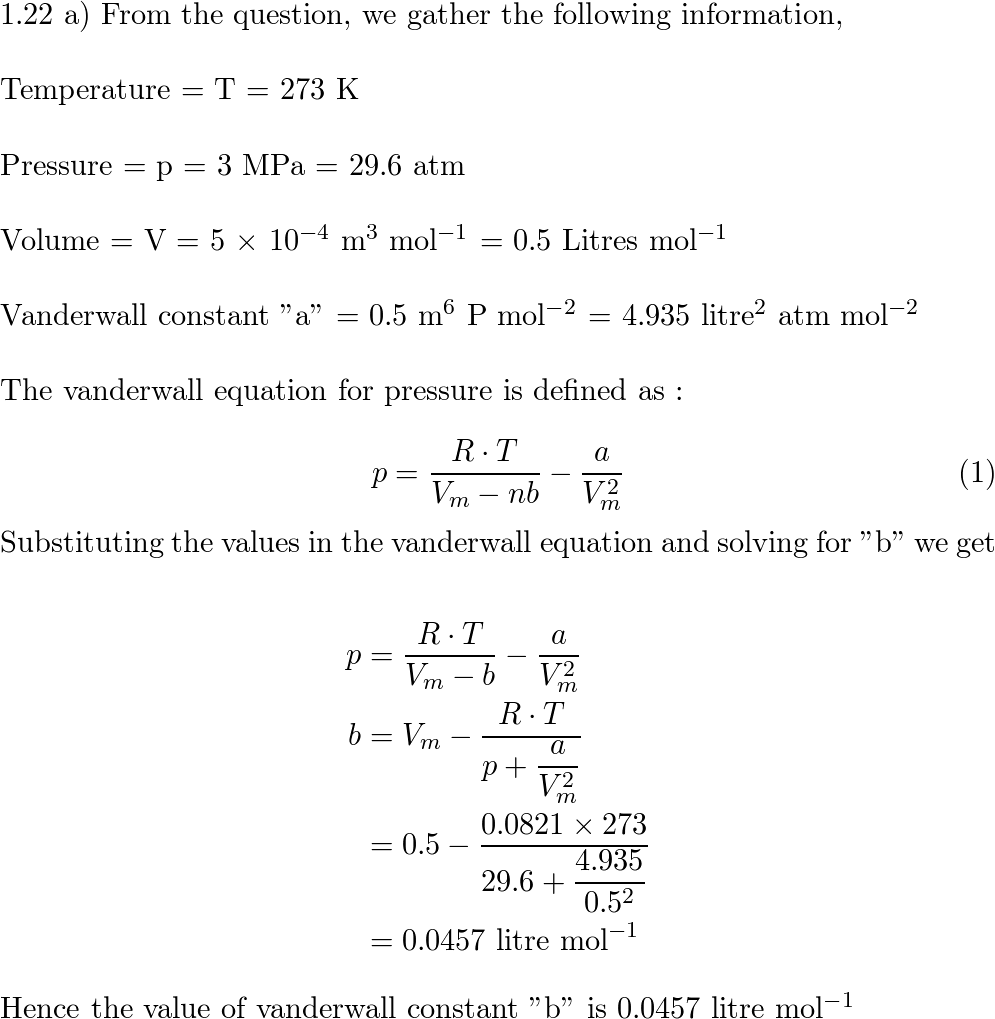
a) A certain gas obeys the van der Waals equation with $a =

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

Solved 1C.9(b) A certain gas obeys the van der Waals
- Excel Calculations: Compressibility Factor for Natural Gas
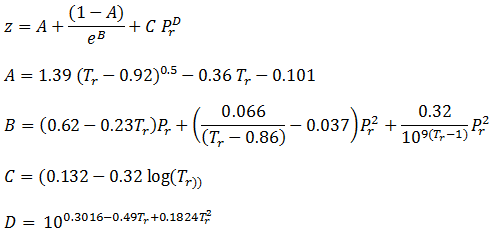
- At certain states, the p-v-T data of a gas can be expressed
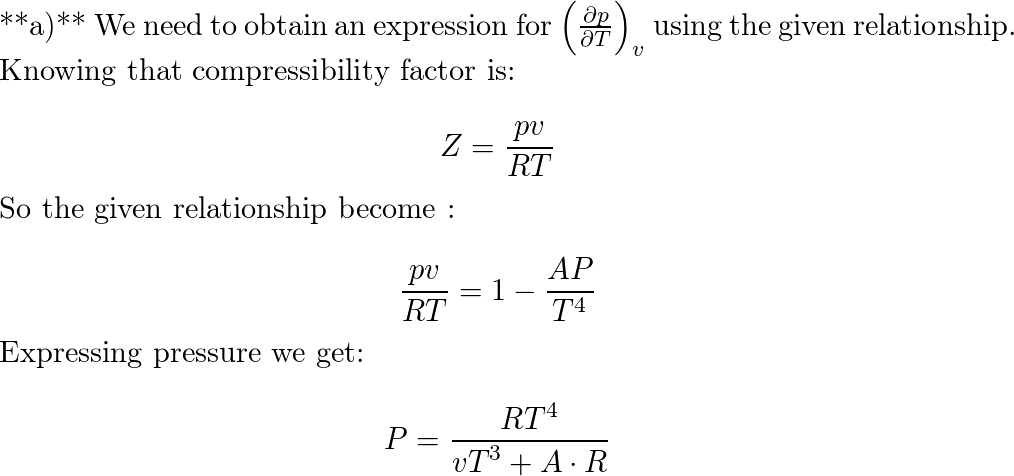
- Which of the following statements is/are correct? (a) all real gases are less compressible

- Procedure calculates base gas compressibility factors
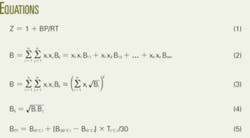
- 1.7: Connecting the van der Waals and the viral equations: the

- AVENUE BODY | Women's Plus Size Full Coverage Wire Free Bra - beige - 38D
- Curvy Couture Women's Sheer Mesh Unlined Underwire Bra Bark 36G
- 2023 Summer Linen Pant Sets Women Elegant Fashion Casual

- Belgian Military Surplus White Briefs, 6 Pack, New - 715860, Military Underwear & Long Johns at Sportsman's Guide

- Best Deal for allgobee Women's Invisible Seamless Panty Non-Trace

- LA SENZA (estair) DIVA COLLECTION, 34C 36B
- Vertical Stripe Tights

- AherBiu Winter Fleece Jumpsuits for Women Plus Size Half Zip up

- Women's Shiny Oily Shimmer Dance Pants High Waist Push Up Leggings Gym Yoga Slim Pants

- Understanding Layers in PDF Sewing Patterns - Pattern Emporium