Compressibility factor (Z) for a van der Waals real gas at

By A Mystery Man Writer
Share your videos with friends, family and the world
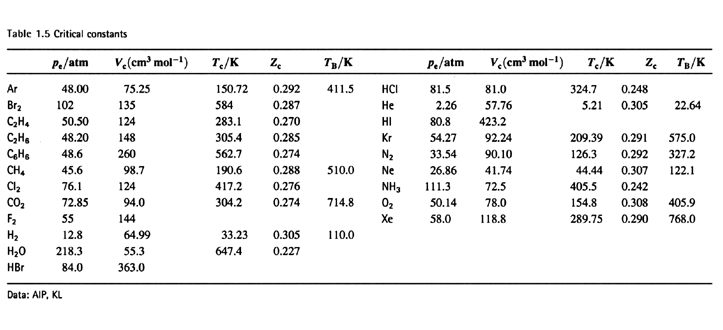
Chapter 1 Properties of Gases
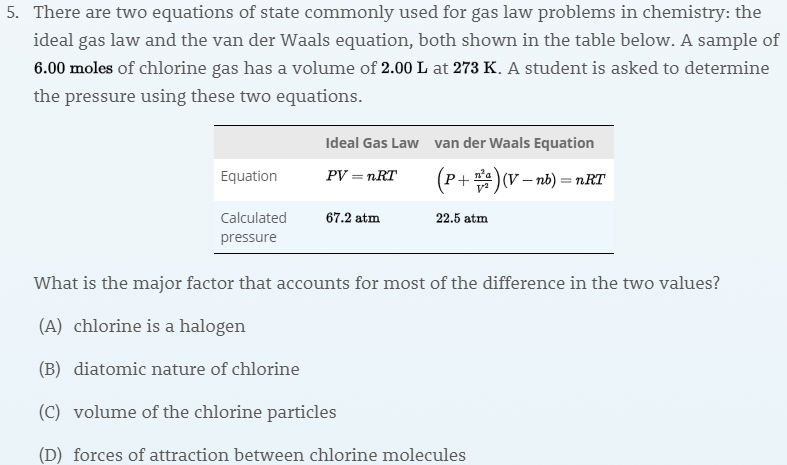
What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?
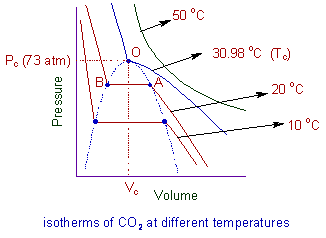
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Solved RT B 2. The compressiblity factor for a gas is

temperature increases at constant a and b values

Compressibility Factor Z Important Concepts and Tips for JEE Main

Van der Waals equation - Wikipedia
COMPREHENSION_TYPE from IIT-JEE PREVIOUS YEAR (CHEMISTRY) STATES OF MATTER for Class 12

Solved Real gas effects can be expressed as departures from
PPT - Real gases PowerPoint Presentation, free download - ID:3959491

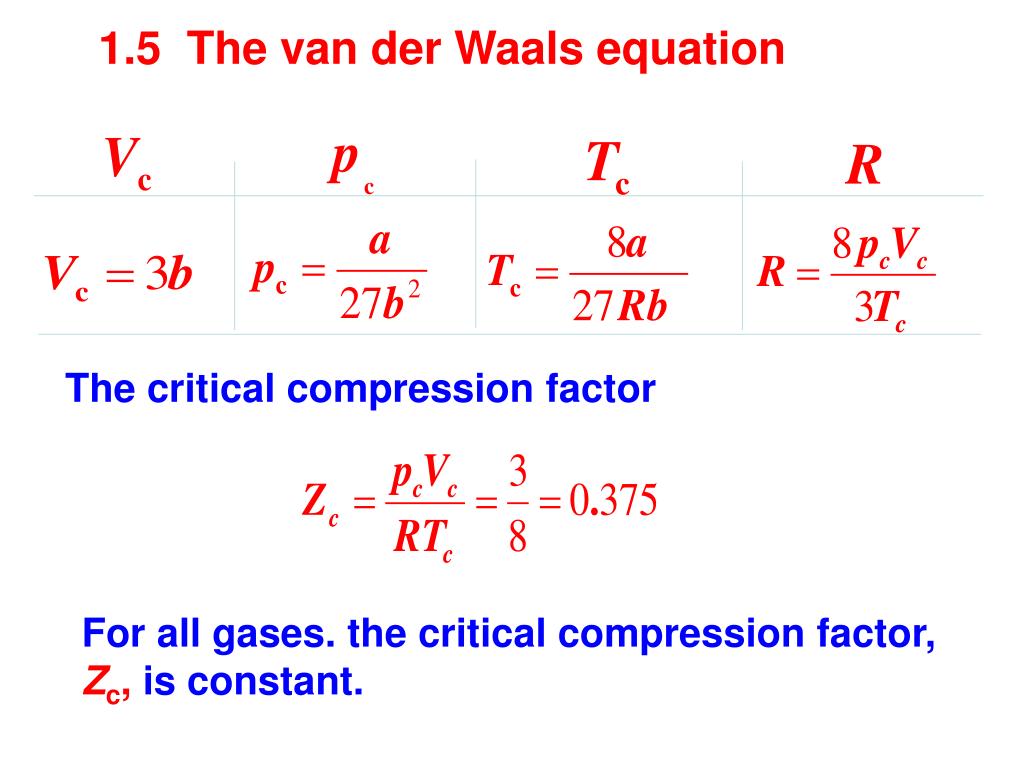
The compressibility factor of a van der Waals gas the critical point is equal to
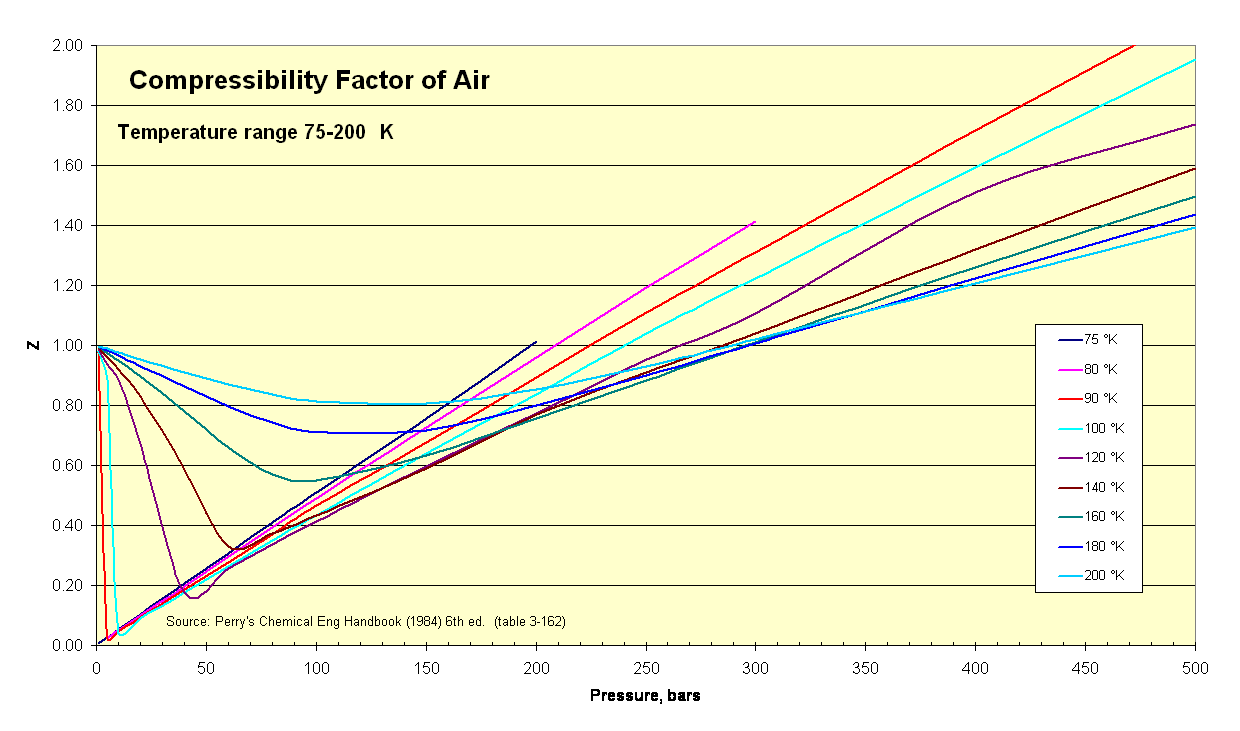
- Air Compressibility Factor Table - EnggCyclopedia
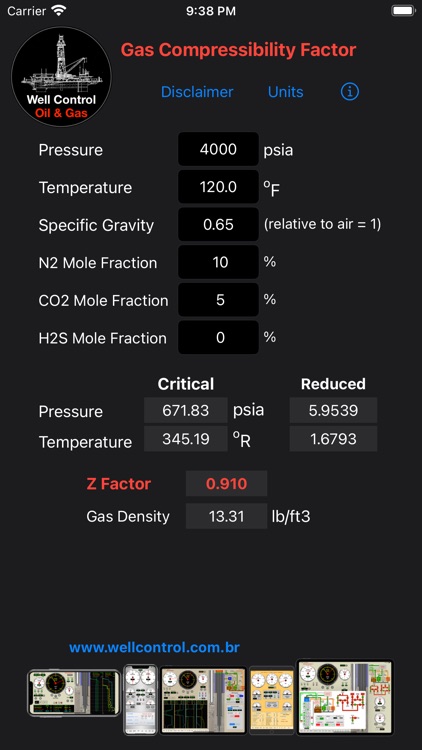
- Gas Compressibility Factor Z by Carlos Moura
- Compressibility factor Z for sub-critical pressures in a 'one-cell

- The compressibility factor Z a low-pressure range of all gases

- physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange