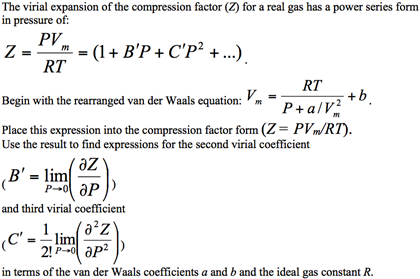
Real Gases. The ideal gas equation of state is not sufficient to

By A Mystery Man Writer
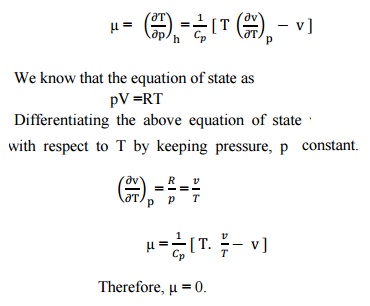
Most real gases depart from ideal behaviour at deviation from low temperature high pressure.
High positive potential energy (little separation) Repulsive interactions Intermediate separations attractive interactions dominate Large separations (on the right) the potential energy is zero and there is no interaction between the molecules..
Real gas molecules do attract one another (P id = P obs + constant) Real gas molecules are not point masses (V id = V obs - const.)
V id = V obs - nb b is a constant for different gases P id = P obs + a (n / V) 2 a is also different for different gases Ideal gas Law P id V id = nRT
Critical temperature (T c ) - the temperature above which a gas cannot be liquefied Critical pressure (P c ) – the minimum pressure that needs to be applied at T c to bring about liquefaction
For a perfect gas, the slope is zero Boyle temperature the slope is zero and the gas behaves perfectly over a wider range of conditions than at other temperatures.
Boyle temperature - for a van der Waal s gas, the Boyle temperature (T B ) is written
The reduced state variables are defined
Re-write the Van der Waals in terms of reduced variables
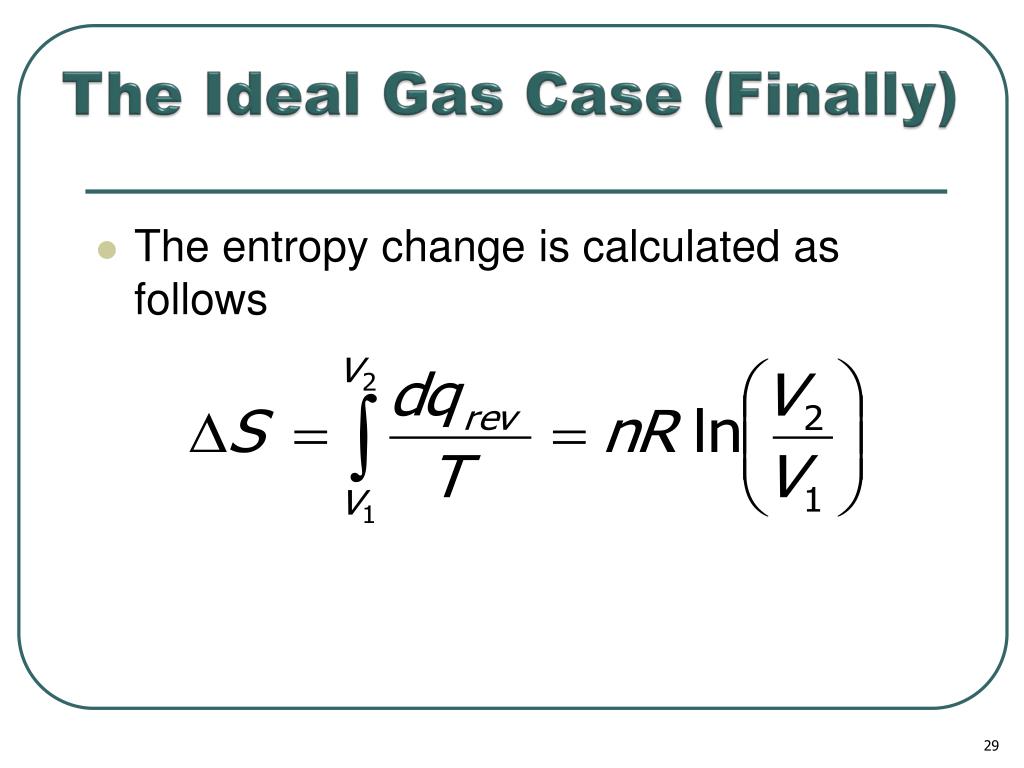
The chemical potential of a real gas is written in terms of its fugacity
In gaseous systems, we relate the fugacity (or activity) to the ideal pressure of the gas via.
Define the fugacity coefficient = f / P For a real gas.
Comparing the chemical potential of the real gas to the chemical potential of an ideal gas at the same pressure
The fugacity coefficients are obtained from the compression factors (Z) as shown below

Comparison of ideal and real gases

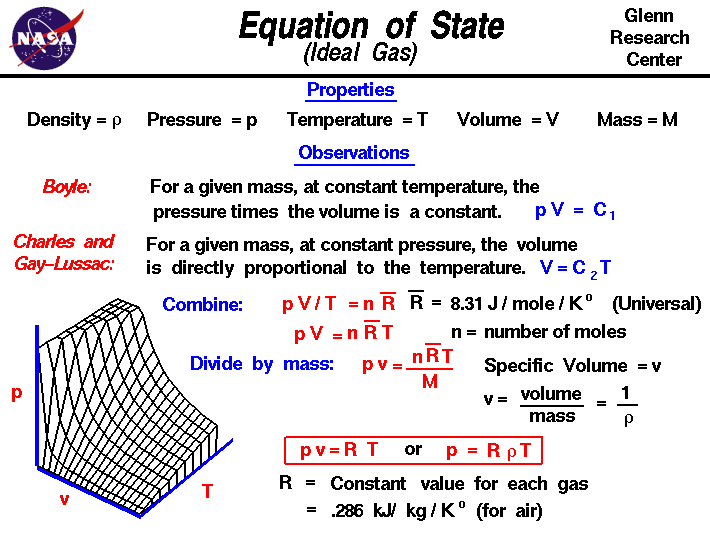
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas
PPT - Chemistry 231 PowerPoint Presentation, free download - ID:917796

Real Gases. The ideal gas equation of state is not sufficient to
Important Questions and Answers: Ideal And Real Gases

PPT - KMT, Graham's Law & Real Gases PowerPoint Presentation - ID:942282

Deviations from the Ideal Gas Law and Chemistry in the Atmosphere Chemistry 142 B Autumn Quarter, 2004 J. B. Callis, Instructor Lecture # ppt download
Equation of State
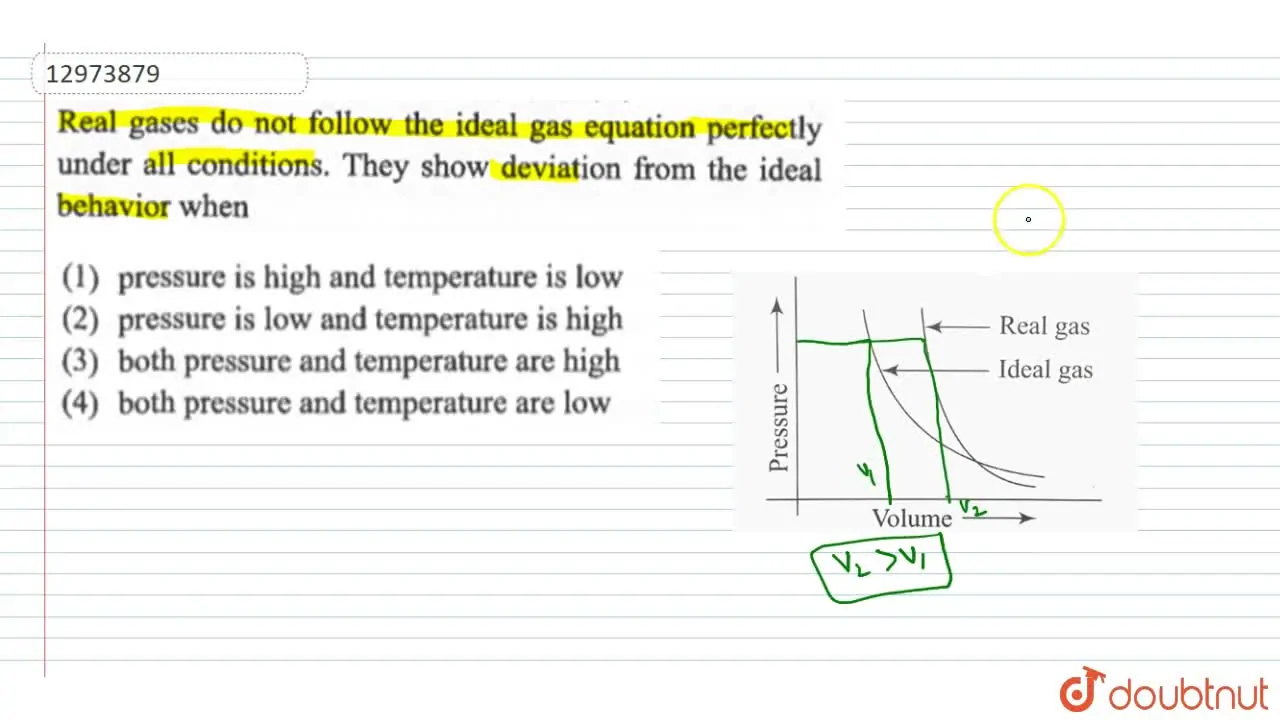
both pressure and temperature are high

PPT - Ideal Gases : PowerPoint Presentation, free download - ID:2757596

Gas Laws For Real Gases. - ppt download
Deviation of Gas from Ideal Behaviour and Its Causes

What is the Maxwell-Boltzmann distribution? (article)
- LANREN Plus Size C D Cup Women Bra Lace Ultra-Thin Lace Perspective Intimates Lingerie and Underwear (Bands Size : 38-85 C D, Color : Style2-red) : Clothing, Shoes & Jewelry

- Anzac Day: Special recording of the Last Post, Reveille to play

- Essentials Women's Full Coverage Minimizer

- TEVEO Focus Scrunch Leggings Schwarz Größe S in Leipzig - Leipzig, Zentrum
- Girls Bell Bottom Flare Pants Cute Girls' Clothes – Hayden Girls