An ideal gas initially P_i ,V_i , and T_i is taken through a cycle

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:an ideal gas initially at pi vi and ti is taken through a cycle
Click here👆to get an answer to your question ✍️ An ideal gas initially P-i -V-i - and T-i is taken through a cycle as shown in Figure- -a- Find the net work done on the gas per cycle 1-00 mol of gas initially 0-0C- -b- What is the net energy added by heat to the gas per cycle
Solved] An ideal gas described by Ti = 275 K, Pi = 1.10 bar, and Vi = 10.0

Solved An ideal gas initially at Pi, V;, and T; is taken
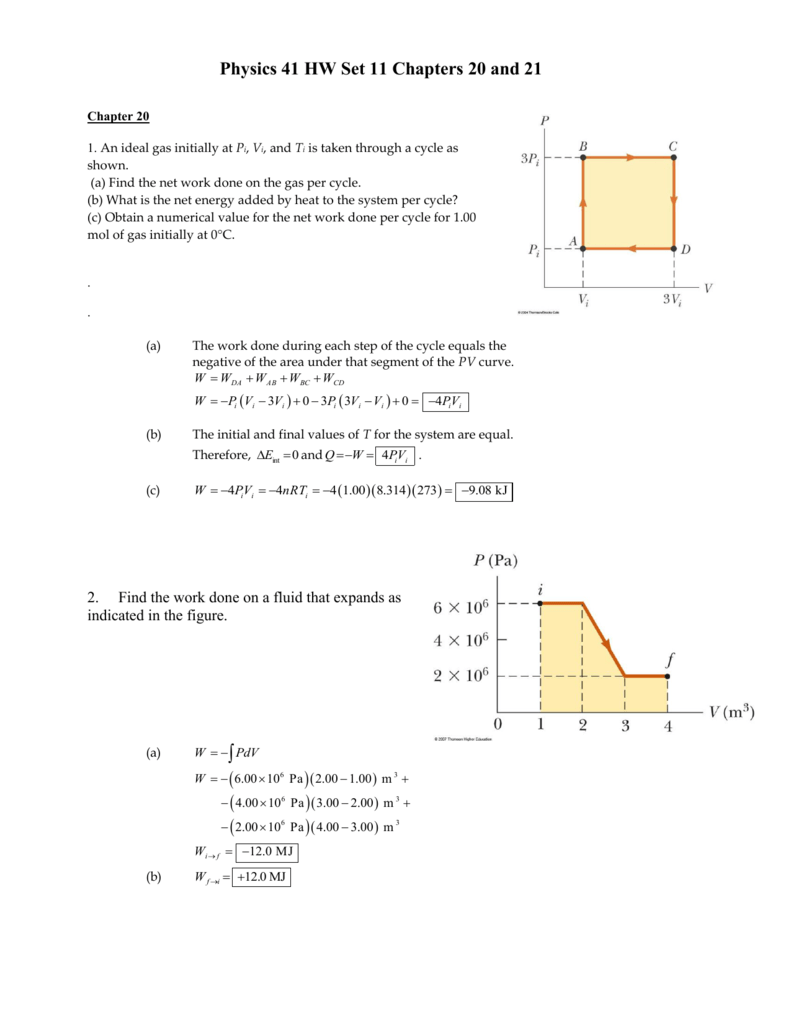
Physics 41 Chapter 21 HW Set 1

An ideal gas expands isothermally from volume `V_(1)` to `V_(2)` and is then compressed to original

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R

Dynamics of ice formation in the Upper Niagara River

Ideal Gas Law and Laws of Thermodynamics, PDF, Gases
Carnot cycle hi-res stock photography and images - Alamy

Progress in interfacial solar steam generation using low-dimensional and biomass-derived materials - ScienceDirect

Materials, Free Full-Text
- Pi Day Gift Tag, Printable Pi Day Card for Teachers, Pi Day Idea for Kids, Pi Day Humor, Pie Gift Tag, Pi Day Party,pi Day Activity,pta PTO

- Unleashing Creativity with Raspberry Pi: A World of IoT Possibilities

- Lipoelastic PI Perfect Post Surgery Bra - Natural

- a) Ideal phase inverter. (b) Configuration of a DSPSL PI (top

- The Ideal Timing for Pi Network Mainnet Launch: Selecting Dates





