Solved 14. The latent heat of vaporization of isopropyl

By A Mystery Man Writer
Answer to Solved 14. The latent heat of vaporization of isopropyl
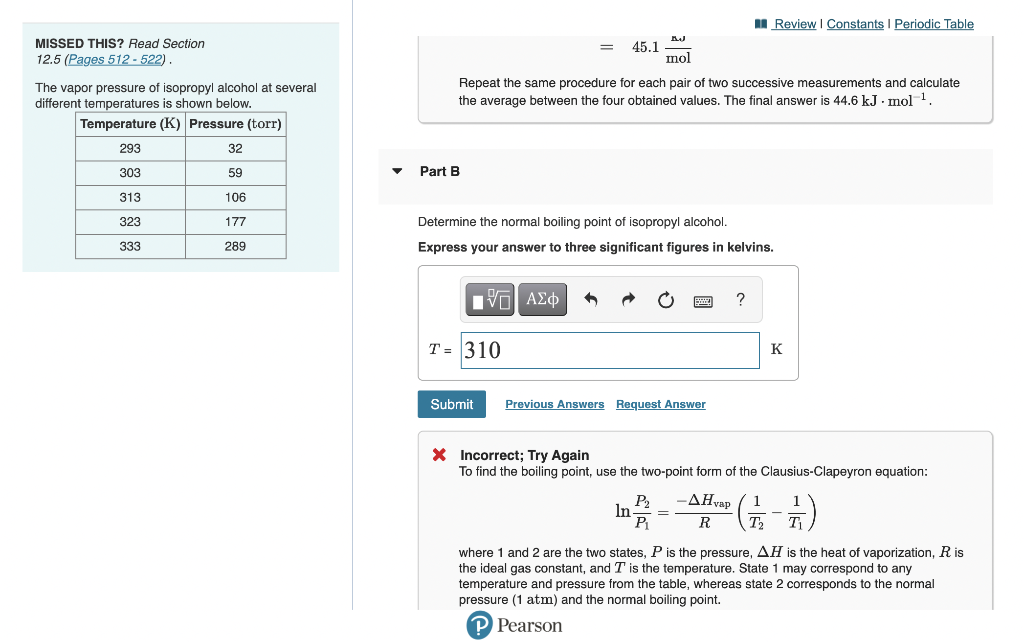
Solved I determined the heat of vaporization to be 44.6

vapor pressure - Why is latent heat of vaporization not exactly proportional to boiling point? - Chemistry Stack Exchange
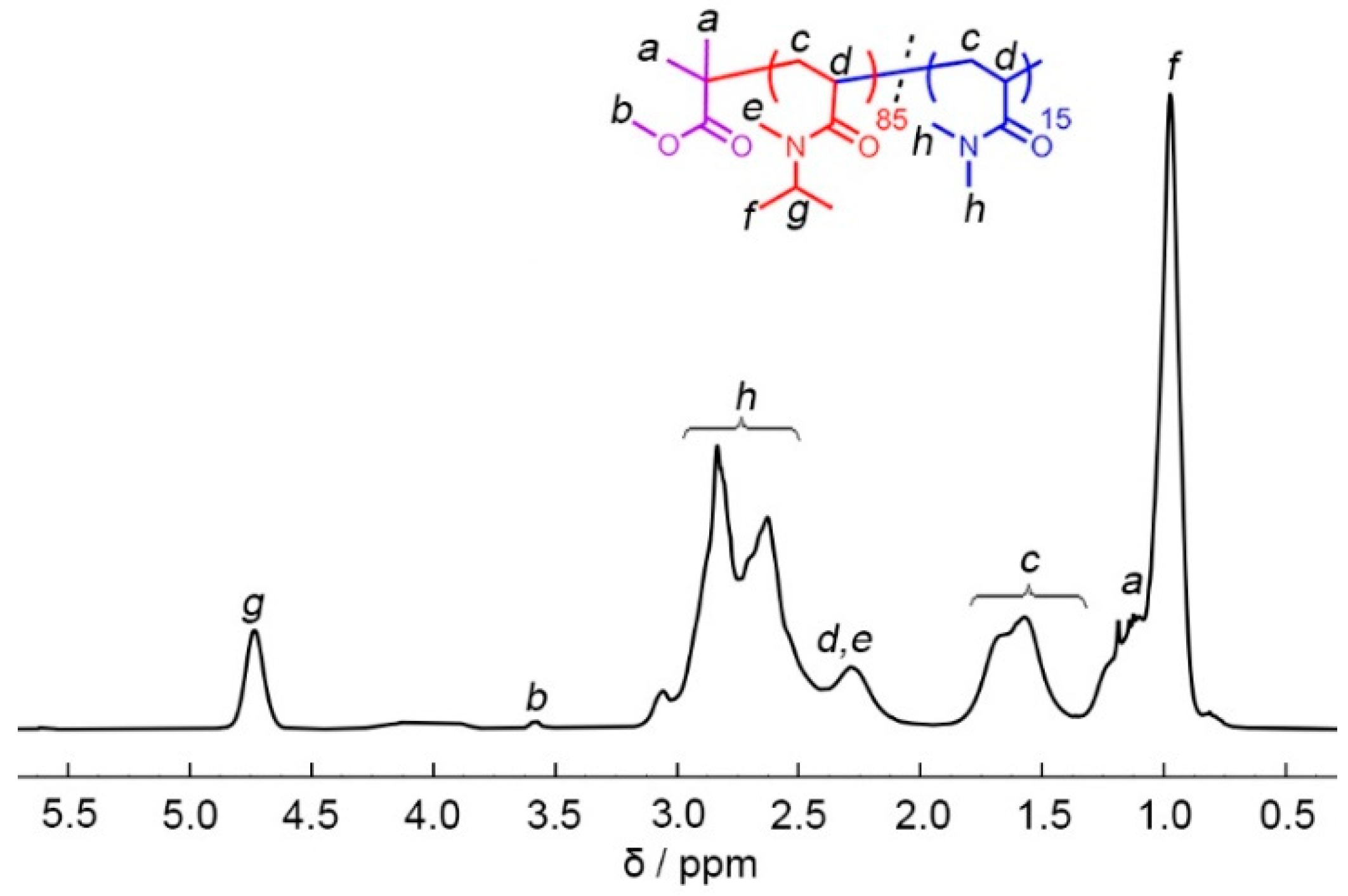
Polymers, Free Full-Text
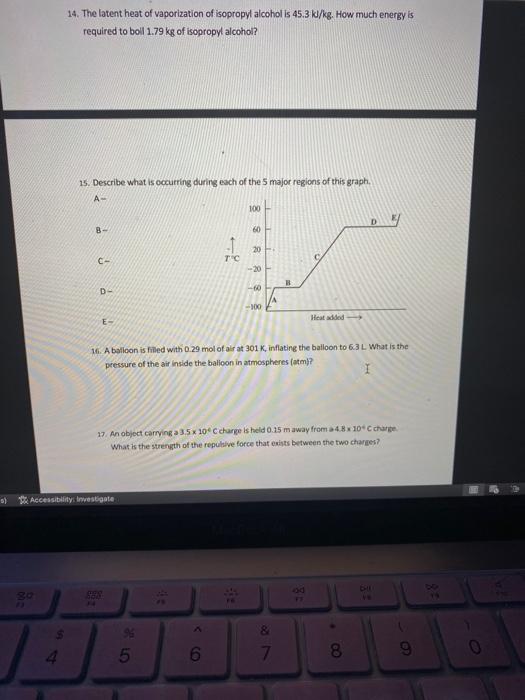
Solved 14. The latent heat of vaporization of isopropyl

Applied Sciences, Free Full-Text

SOLVED: Isopropyl alcohol has a heat of vaporization of 3.99 * 10^3 mol^-1 and a boiling point of 82.30 °C at 1.000 atm. Using the Clausius-Clapeyron equation, calculate the vapor pressure of

SOLVED: Determine the total amount of heat released (in kJ) when 485 g of isopropyl alcohol (C3H8O) are cooled from the gas state at 100 °C to liquid at -42.0 °C. The

Solved 14. The latent heat of vaporization of isopropyl
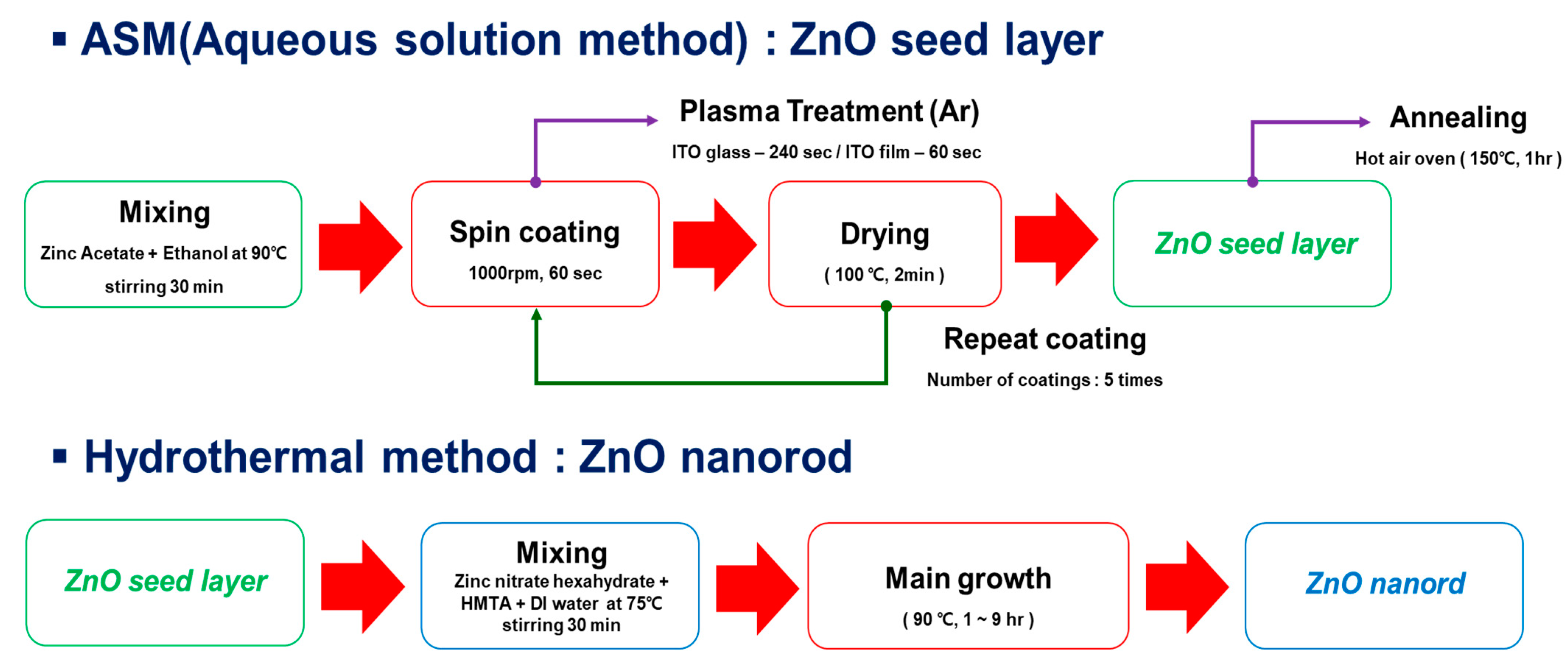
Materials, Free Full-Text
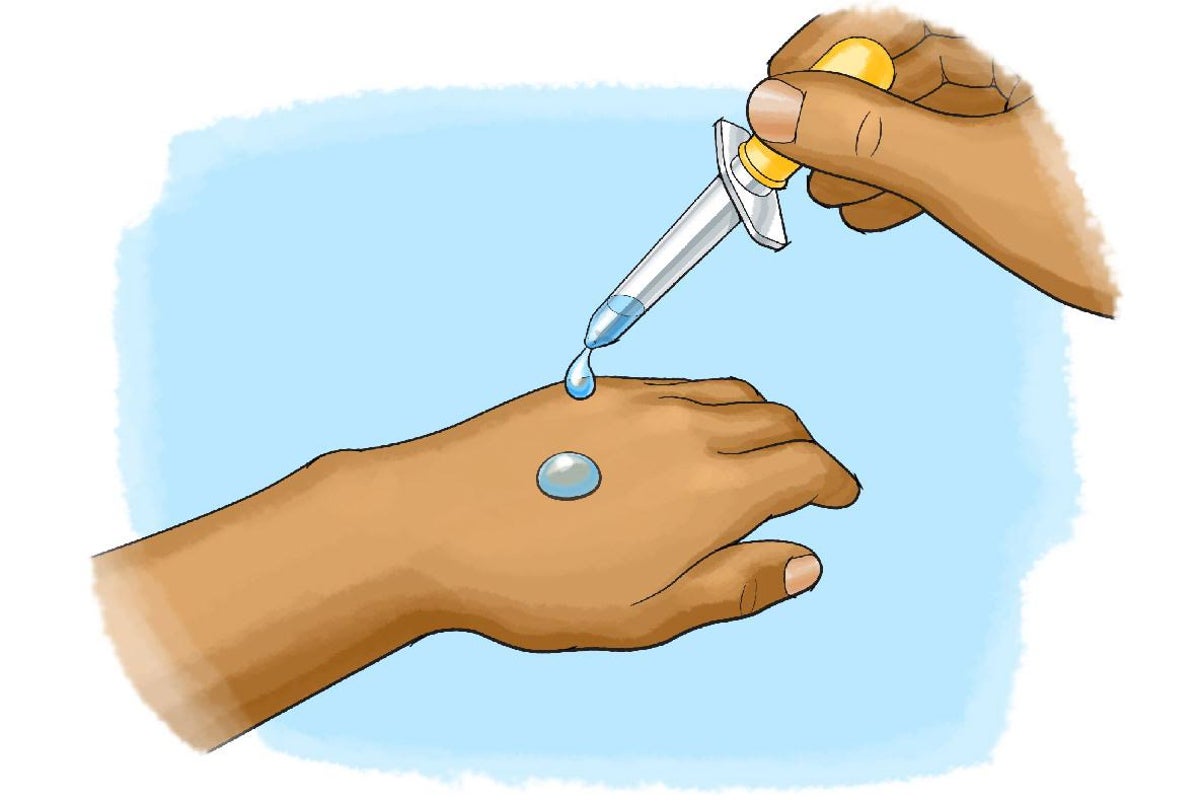
Chilling Science: Evaporative Cooling with Liquids

Materials Proceedings, Free Full-Text
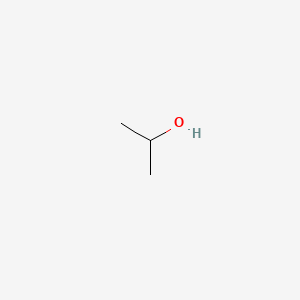
Isopropyl Alcohol, (CH3)2CHOH

Solved Question #: 5 1 grams of isopropyl alcohol
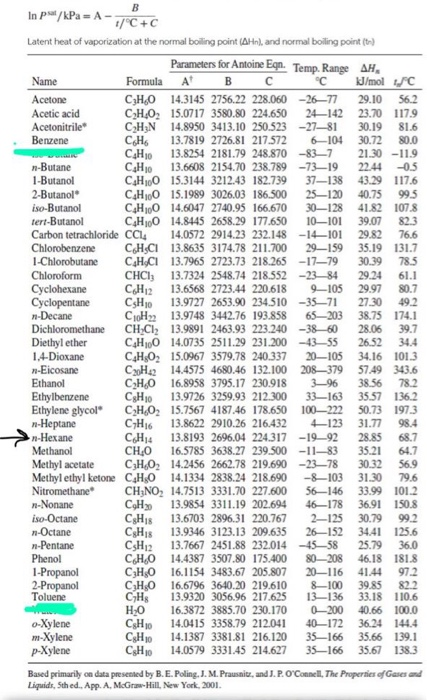
Solved Binary system 1-chlorobutane/chlorobenzene conforms
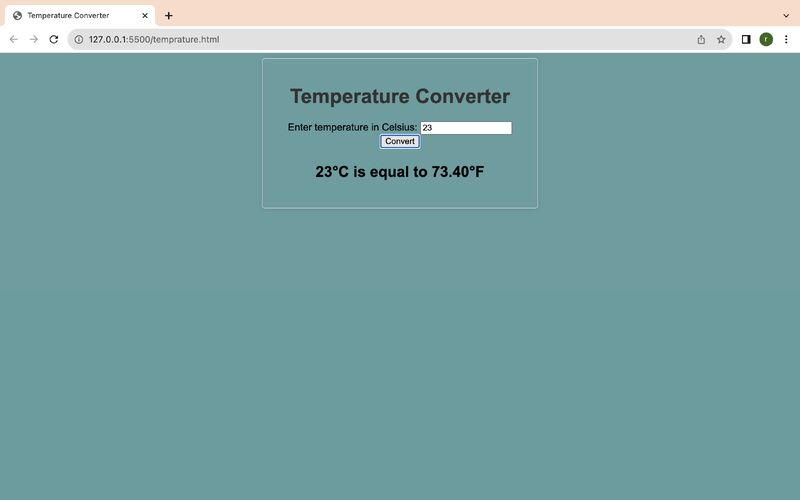
- Launching Temperature Converter website using HTML and CSS
- Convert 23°C to degrees Fahrenheit. If necessary, round your
- Unit 2: Length, Area and Volume Chapter 3 in workbook. - ppt download

- Ch 25 ppp, with breakouts

- 12th Grade Physics: Temperature Conversion) I don't get what the question is asking me to find on number 2. : r/HomeworkHelp




)
