Preparation of Standard Solution of Sodium Carbonate - Chemistry

By A Mystery Man Writer
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU'S to understand more about it.

Chp.13 - Volumetric analysis (acids/bases) [all] Flashcards

Titration Lab Report - Ap0304 Practical Transferable Skills

Calculating molarity units molar concentration of solutions

SOLVED: Task I: Preparation of standard solutions Purpose: To

To prepare 0.1 M standard solution of sodium carbonate in 250ml

LAB Eport titration - Objectives 1. To learn how to prepare a

Explain the commercial method of preparation of sodium carbonate.

Prepare 100 cm3 of 0.1M sodium carbonate (Na2CO3) solution

PDF) Chemistry Experiment Laboratory Report (1) Title

Conversion of gaseous effluents of power plant to sodium carbonate

Sodium carbonate-bicarbonate eluent solution 3 mM Na₂CO₃/2 mM NaOH

Sodium carbonate concentrate Na2CO3 0.1M water, eluent concentrate

Standard solution, Resource

To prepare the 250ml of N/10 solution of sodium carbonate
Preparation of Standard Solution of Sodium Carbonate: Theory
- Corn Cob Bodycon Dress Women Cosplay Halloween Costume Tank Fitted Stretchable Spandex Party Outfit
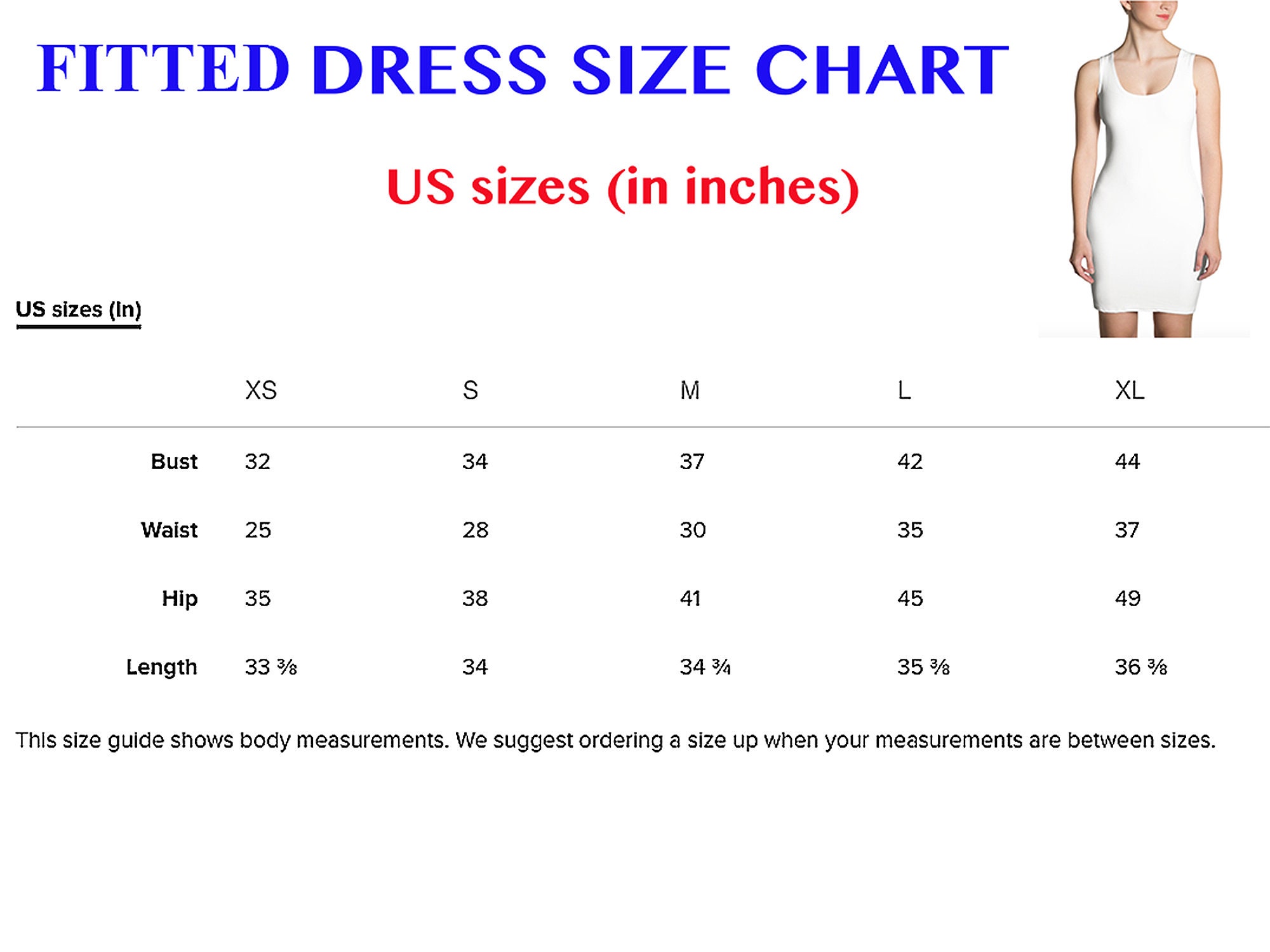
- What Size Am I Women's Calculator - Cuddl

- What is Minimum Blank Size (MBS) and How Do You Calculate It

- Why does counting digits after the decimal point work when multiplying, Mathematics

- How to determine the appropriate sample size for structural equation modeling - Statistics Solutions