Wednesday, Sept 25 2024
The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

By A Mystery Man Writer
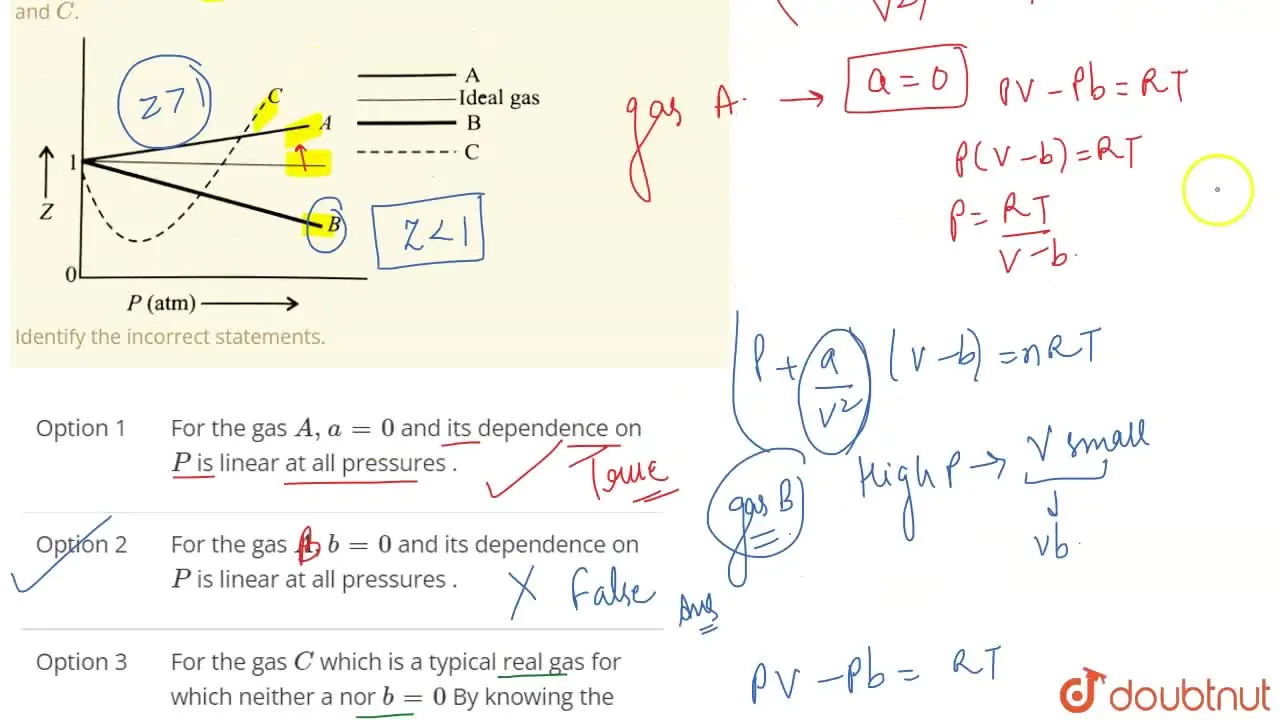
For the gas C which is a typical real gas for which neither a nor b =0
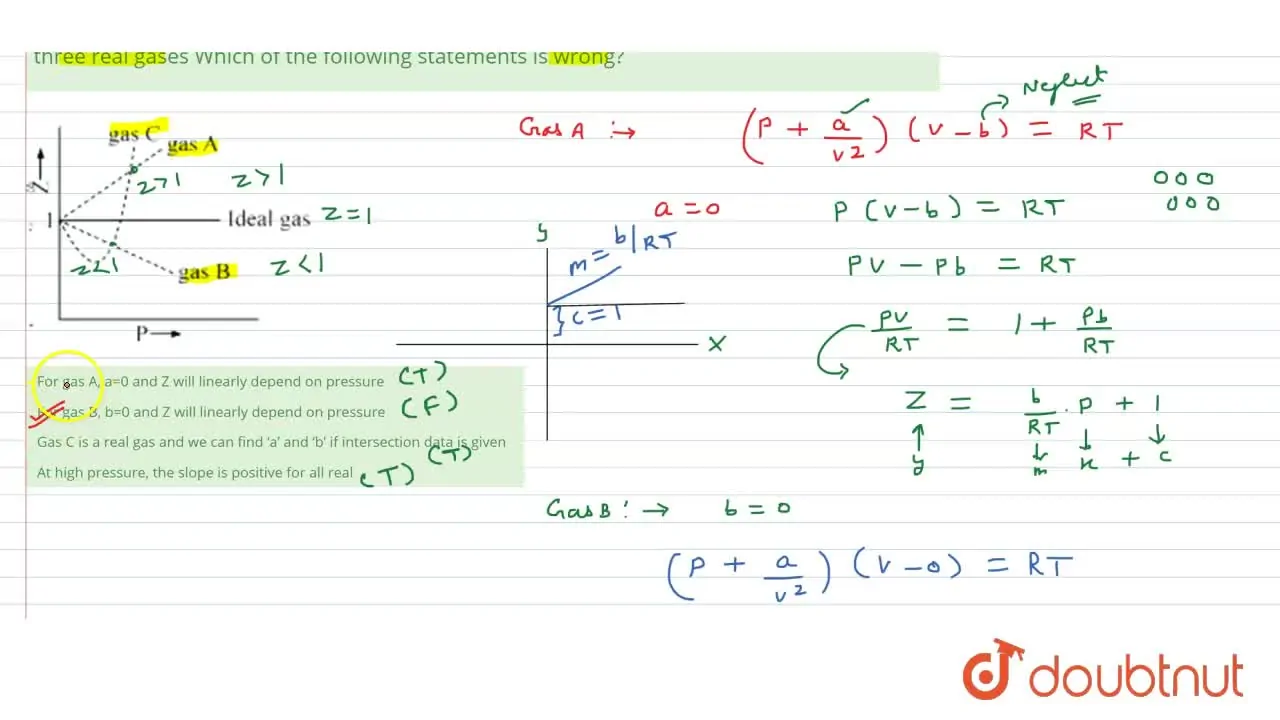
Gas C is a real gas and we can find 'a' and 'b' if intersection data i
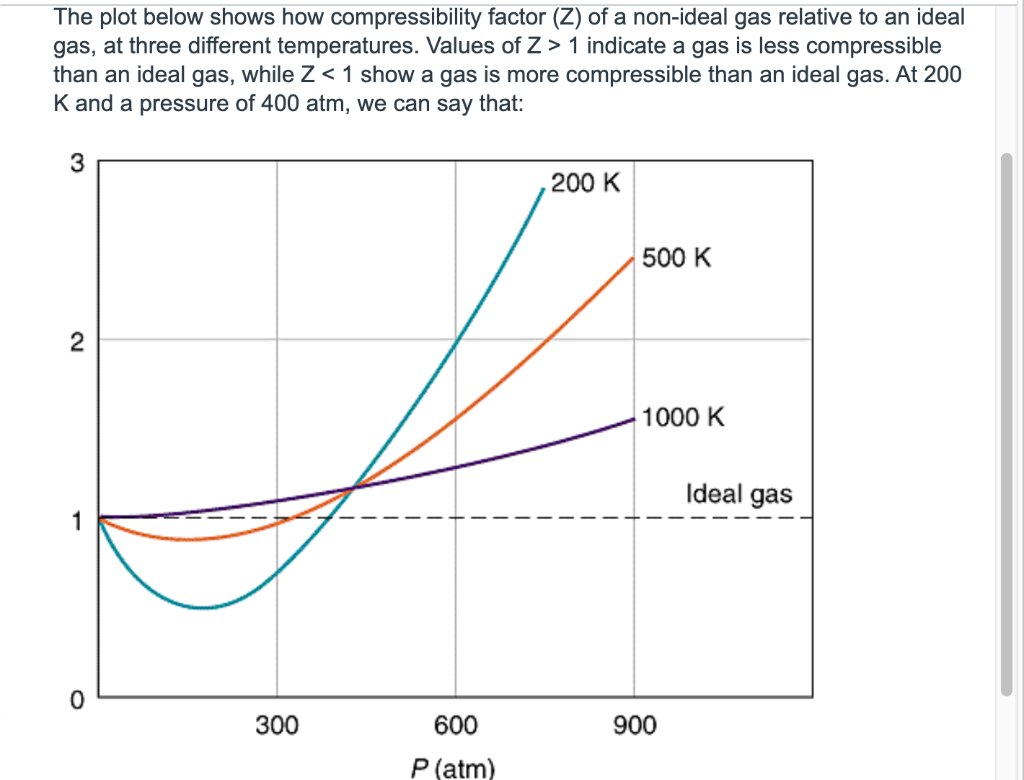
Solved Using the Maxwell-Boltzmann distribution curves

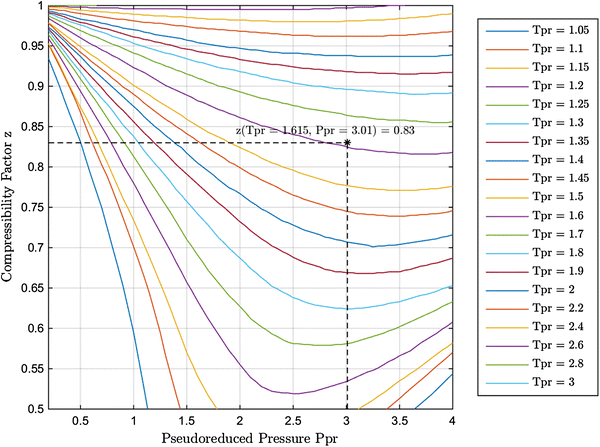
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

Gas C is a real gas and we can find 'a' and 'b' if intersection data i
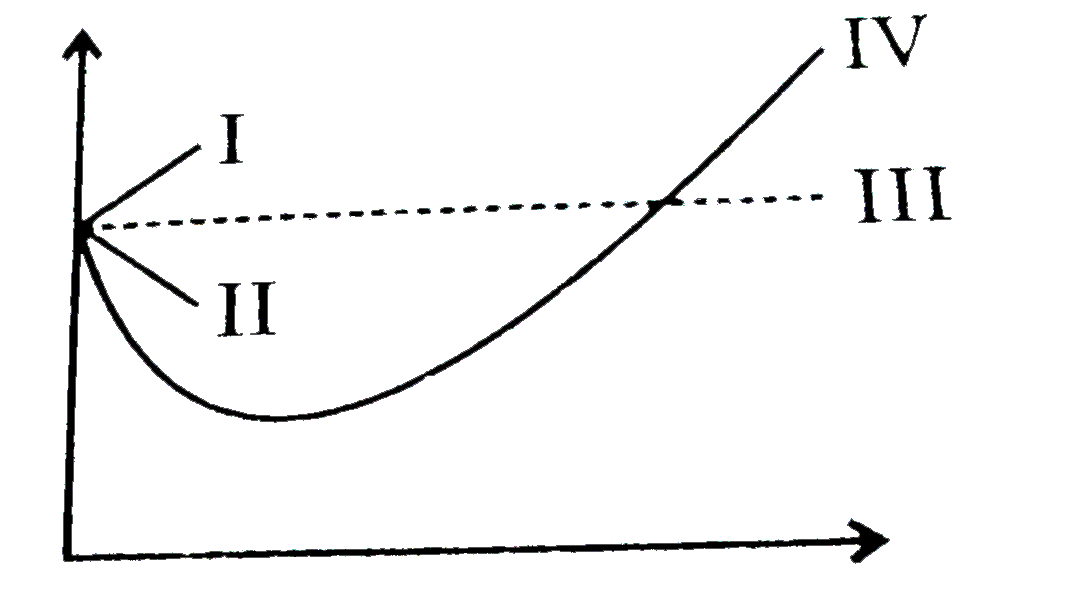
Telugu] The variation of compressibility factor (Z) with pressure (p
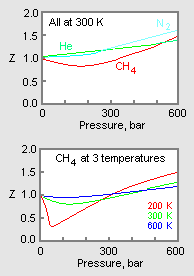
Compressibility factor (gases) - Knowino
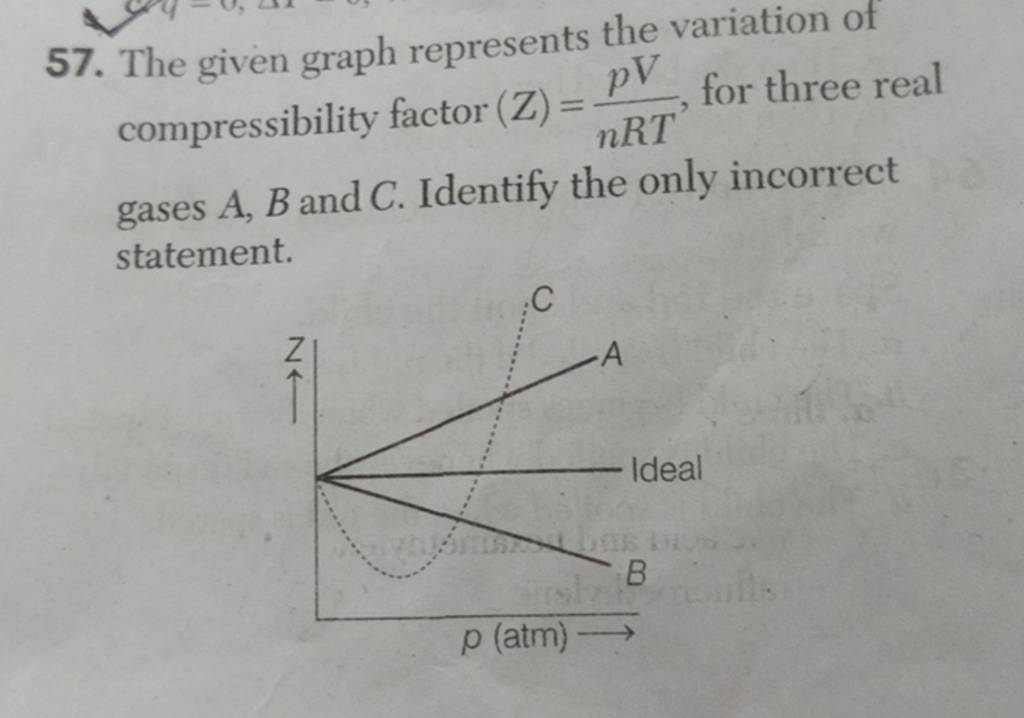
57. The given graph represents the variation of compressibility factor (Z..
Related searches
Related searches
- CAMISETA TIE DYE MANGA CURTA MASCULINA BORA BORA OKDOK 1220326 - CAMISETA TIE DYE MANGA CURTA MASCULINA BORA BORA OKDOK - OKDOK

- Womens Track Shorts Shorts Sporty Drawstring Waist High Waist Light Grey XS

- Logirlve Comfort Seamless Bra And Panties Backless Wireless Set Underwear For Girls Push Up Bra Lace Women Lingerie Set Color Blue Cup Size 85A

- Kliou Mesh Print Foot Pants Women Medium Waist Skinny Body-shaping

- Kayak Malzemeleri Nelerdir? - Snowboard Malzemeleri Nelerdir? - Kamp Yerleri

©2016-2024, sincikhaber.net, Inc. or its affiliates