What is the value of compressibility factor in terms of vander
By A Mystery Man Writer
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas
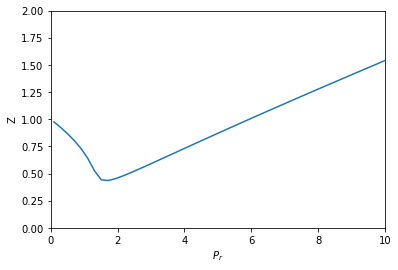
Compressibility factor variation from the van der Waals equation by three different approaches

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

Gas Compressibility Factor and Control Valve Sizing

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

The compressibility factor in terms of Pc, Vc and Tc is called Zc. Th

The role of the compressibility factor Z in describing the volumetric behavior of gases
How I find the a and b constant in the Van der Waals equation? - Quora
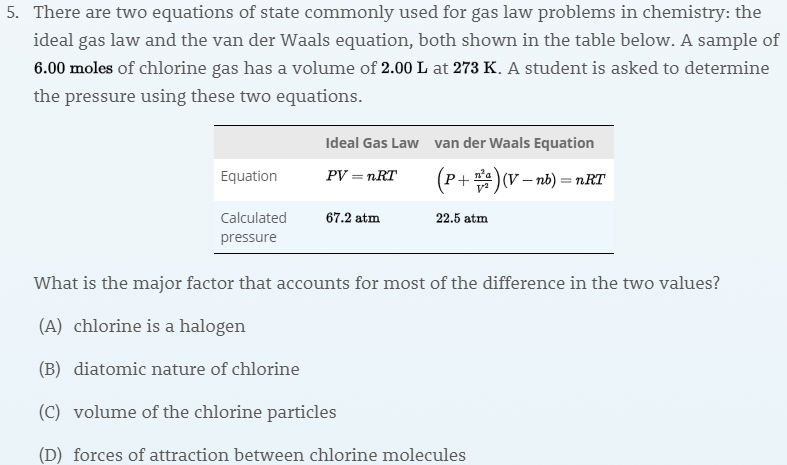
What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?

6.3: Van der Waals and Other Gases - Physics LibreTexts

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very

Compressibility Chart - an overview

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

Compressibility factor for methane.








