Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism, Catalysis, ChemRxiv

By A Mystery Man Writer
Despite numerous experimental and theoretical studies devoted to the oxygen evolution reaction, the mechanism of the OER on transition metal oxides remains controversial. This is in part owed to the ambiguity of electrochemical parameters of the mechanism such as the Tafel slope and reaction orders. We took the most commonly assumed adsorbate mechanism and calculated the Tafel slopes and reaction orders with respect to pH based on microkinetic analysis. We demonstrate that number of possible Tafel slopes strongly depends on a number of preceding steps and surface coverage. Furthermore, the Tafel slope becomes pH dependent when the coverage of intermediates changes with pH. These insights complicate the identification of a rate-limiting step by a single Tafel slope at a single pH. Yet, simulations of reaction orders complementary to Tafel slopes can solve some ambiguities to distinguish between possible rate-limiting steps. The most insightful information can be obtained from the low overpotential region of the Tafel plot. The simulations in this work provide clear guidelines to experimentalists for the identification of the limiting steps in the adsorbate mechanism using the observed values of the Tafel slope and reaction order in pH-dependent studies.

Mechanistic understanding of pH effects on the oxygen evolution reaction - ScienceDirect

Atomistic Understanding of Two-dimensional Electrocatalysts from First Principles

Microkinetic assessment of electrocatalytic oxygen evolution reaction over iridium oxide in unbuffered conditions - ScienceDirect

Potential-dependent OER performance on dual-Fe-Ir sites by grand canonical based constant charge method - ScienceDirect

Photocatalysis Lecture 2 Basics of Tafel Slope_industry trends-Perfectlight

a) Representative cyclic voltammograms (fifth cycle) of nano-and

Potential-dependent OER performance on dual-Fe-Ir sites by grand canonical based constant charge method - ScienceDirect

DFT calculation of the OER reaction mechanism to elucidate intrinsic

Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism, Catalysis, ChemRxiv

Electrochemical hydrogenation and oxidation of organic species involving water

Electrocatalytic performance of Rh-RuO2/G, RuO2/G, and commercial RuO2

DFT calculation of the OER reaction mechanism to elucidate intrinsic

PDF) Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism

Hydrogen evolution reaction from bare and surface-functionalized few-layered MoS2 nanosheets in acidic and alkaline electrolytes - ScienceDirect
- 6.3: Thermodynamics and kinetics - Engineering LibreTexts
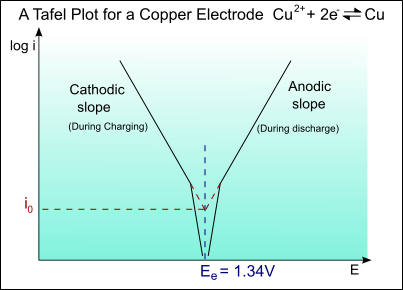
- The Tafel Equation: A guide to Electrochemical Kinetics - Macias Sensors

- Tafel slopes derived from the cyclic voltammetry on stationary
- Bayesian data analysis reveals no preference for cardinal Tafel slopes in CO2 reduction electrocatalysis. - Abstract - Europe PMC

- Comparison of the polarization curves (a) and Tafel slope curves

- Womens Swim Top Large Bust Bikini Split Swimsuit for Women Swimsuit Board Shorts for Women (Black, S) : Clothing, Shoes & Jewelry

- PANTY RESHAPER BLACK

- 5054B Latex Body Strapless Thong – Ann Michell Store

- McKesson Feeding Pump Backpack Black, Pump Pocket, Outside View

- Playtex 18 Hour Ultimate Lift and Support Wireless Bra 4745 - Macy's
