The FDA's rule change requiring providers to inform women about

By A Mystery Man Writer

The FDA's Rule Change Requiring Providers to Inform Women About Breast Density Could Lead to a Flurry Of Questions – ActiveBeat – Your Daily Dose of Health Headlines

Be inforMD: know your breasts

Page 7 – Women's Healthcare

The FDA's rule change requiring providers to inform women about
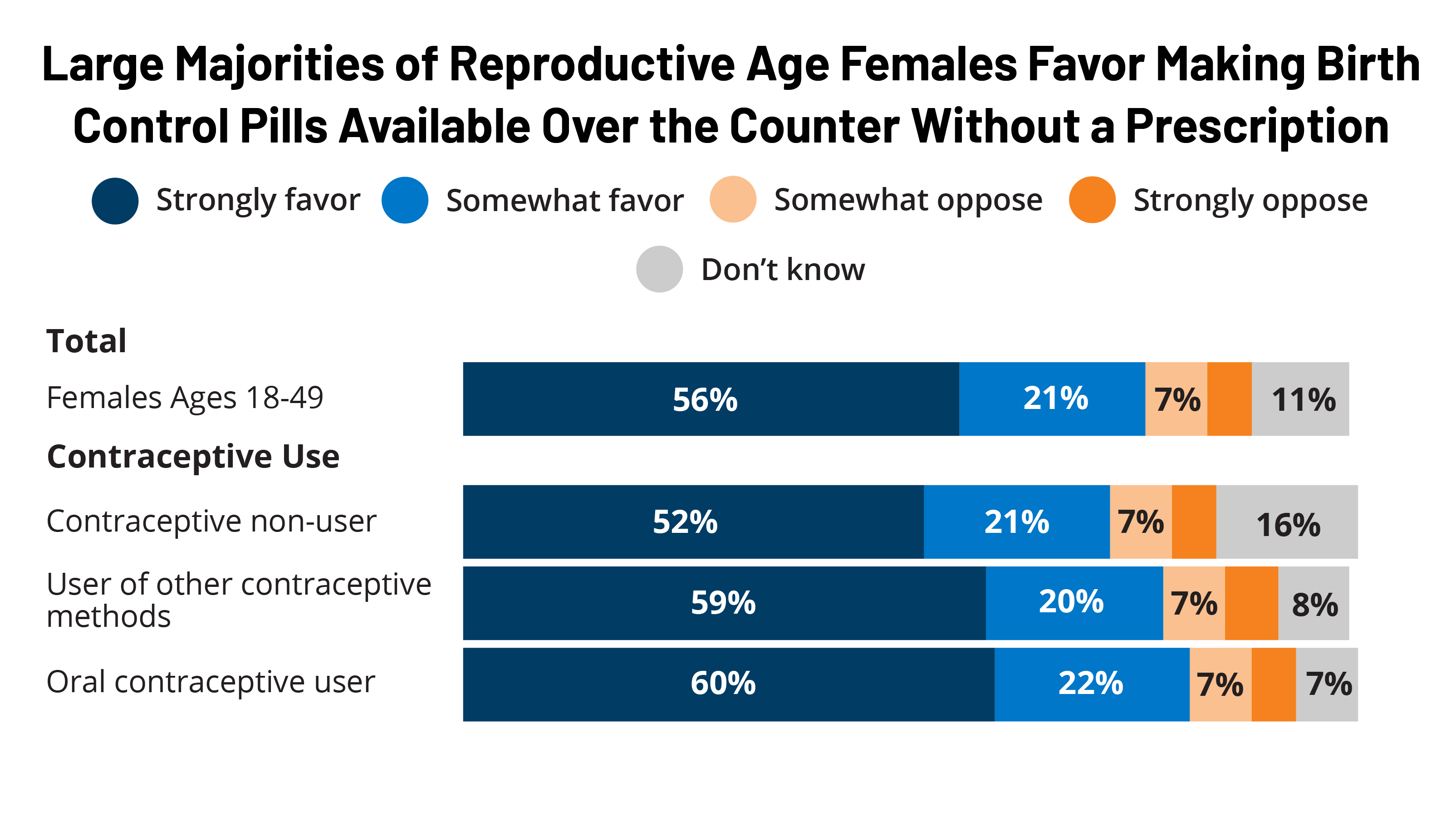
Interest in Using Over-the-Counter Oral Contraceptive Pills: Findings from the 2022 KFF Women's Health Survey

Women's Health Policy

Mammograms – News, Research and Analysis – The Conversation – page 1

FDA to Implement New Mammogram Regulations to Support Women with Dense Breasts

Understanding the FDA's new proposed regulations on human subject research and their impact on your clinical trial plans

Choose Life Marketing

cancer awareness Archives - UPMC & Pitt Health Sciences News Blog
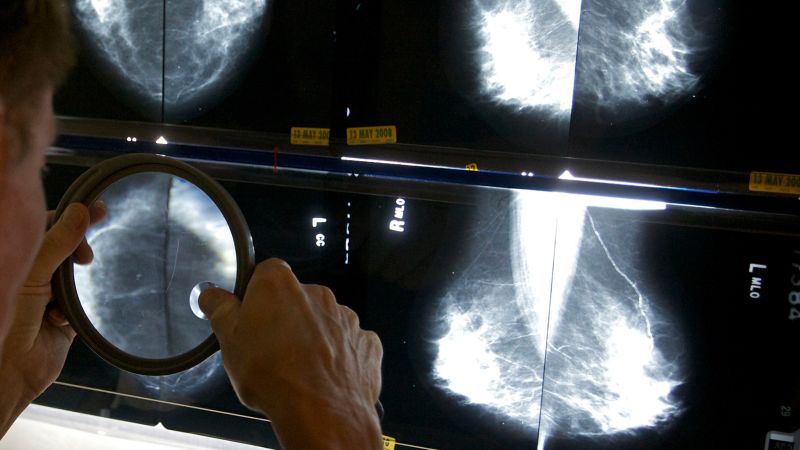
FDA to require mammogram reports include breast density information
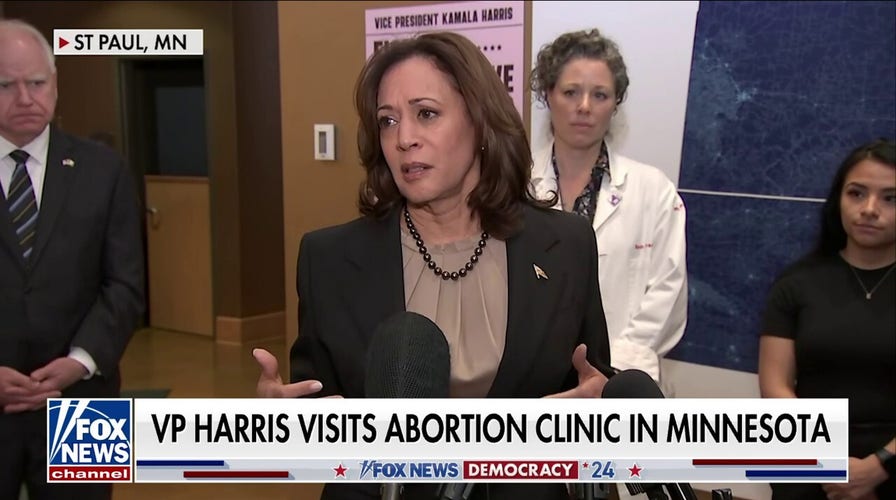
Pro-life groups slam FDA after Supreme Court mifepristone arguments

Women Can Wait Longer Between Pap Tests, Doctor Reveal
- Silicone Breast Form Mastectomy Prosthesis Waterdrop Enhancer One Piece 250g AA Cup

- What is the difference between an A cup bra and an AA cup bra? Why
- BA Journey from a Flat-chested, Less Than AA Cup Girl - Review

- Mini-puberty of infancy in a 16-month-old girl with prominent

- Breast Augmentation - Breast Augmentation Macleod Trail Plastic
- Hanro Luxury Moments Lace Unlined Underwire Bra - ShopStyle

- 1Pack Pre-Twisted Passion Twist Curly Crochet Hair For Women, Black Pre-Looped Passion Twists Synthetic Braiding Hair

- WDIRARA Women's Plus Size Ruffle Trim Elastic Waist Wide Leg Pants Stretch Palazzo Pants Black 0XL at Women's Clothing store

- Ponte Wide-Leg Pant by Jules & Leopold

- m.media-/images/I/71INUF-nMAL._AC_UY1000
