42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g

By A Mystery Man Writer
Share your videos with friends, family and the world

5.2: Reaction Stoichiometry (Problems) - Chemistry LibreTexts
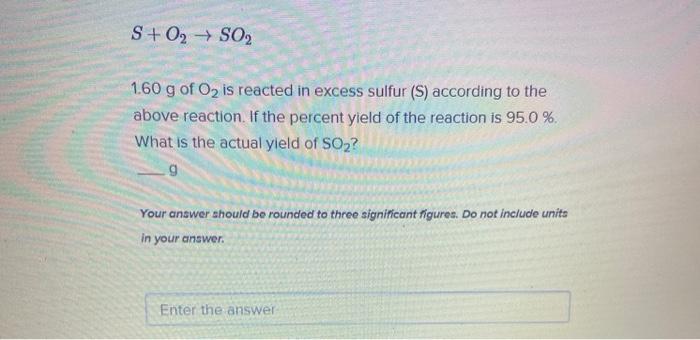
Solved S + O2 + S02 1.60 g of O2 is reacted in excess sulfur

Answered: if 24.0g of NO and 13.8g of O2 are used…

WO2012002527A1 - HETEROCYCLIC COMPOUND, AND p27 KIP1 DEGRADATION

UMAIR KHAN ACADEMY

Topical Mock Chemistry Questions, PDF

Regents Chemistry Exam Explanations June 2019

JP2022531876A - Convergent liquid phase synthesis of
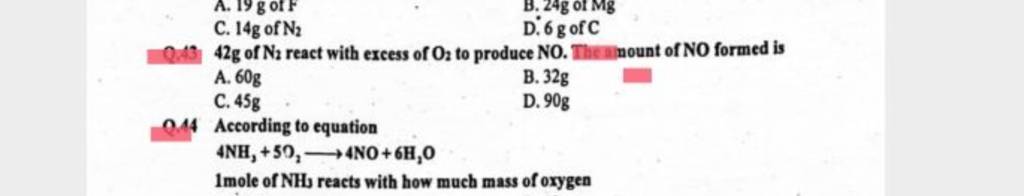
42 g of N2 react with excess of O2 to produce NO. Theninount of
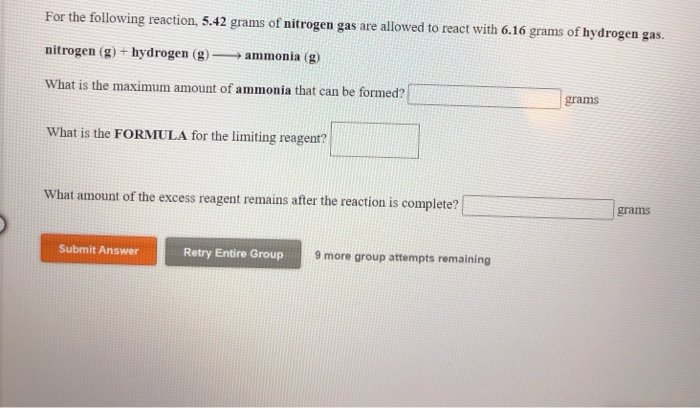
Solved For the following reaction, 10.9 grams of nitrogen

Solutions Dinesh, PDF, Molar Concentration

Solved 21. Consider the following chemical reaction: N2+ O2

WO2022045231A1 - Ester compound - Google Patents

Mole Concept PDF, PDF, Mole (Unit)
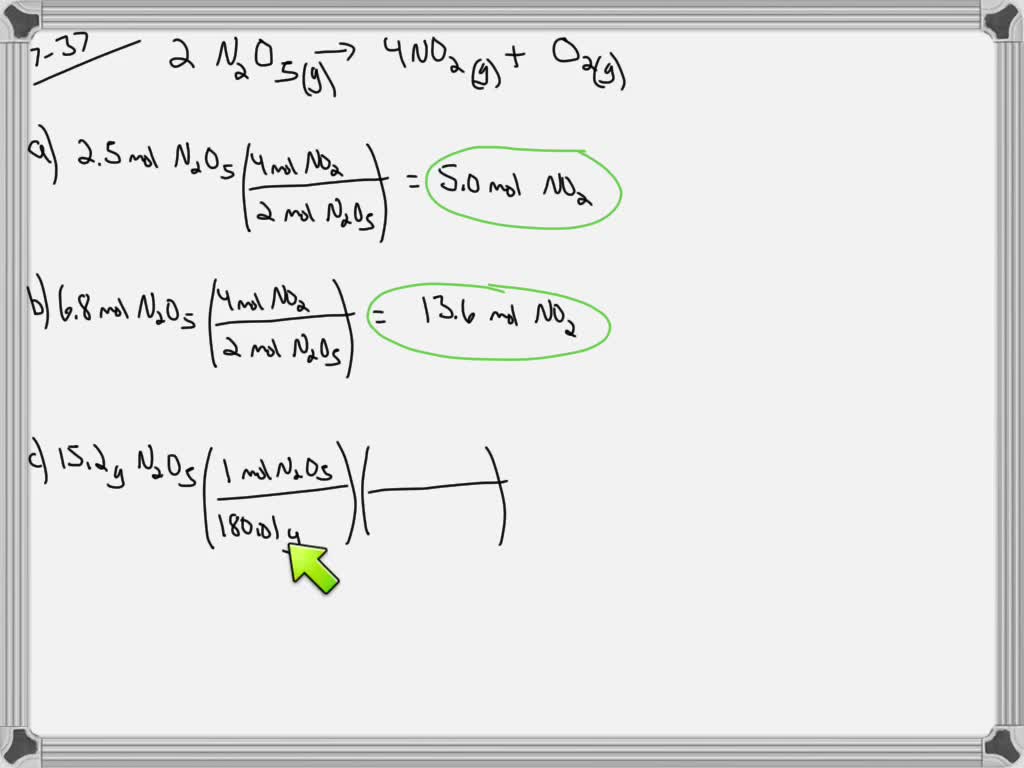
SOLVED: Calculate how many moles of NO2 form when each quantity of reactant completely reacts. 2 N2O5(g)-4 NO2(g) + O2(g) a. 2.5 mol N2O5 b. 6.8 mol N2O5 c. 15.2 g N2O5
- Direct Fuel Level Indicator C-182 42G

- Chips De Vegetais Batata Doce 42g Kit C/ 6 Unid

- Global Gmarket Mobile
- Genuine FIMO® Kids Polymer Modelling Oven Bake Clay 42g * 20 Different Colors

- 10-pack Jwk C Black V2 Lubed Switch Linear 5 Pin 42g Actuation 58.5g Bottom Out For Mx Mechanical Keyboard Hotswap Pcb - Keyboards - AliExpress

- Customization Kids Youth Version 2022-2023 Brazil Home Away Premium Soccer Football Jersey Set

- Buy Inner Sense Soft Organic Cotton Bamboo Triangular Bra for Women I Non Padded, Wire Free Everyday Bra for Women AISB099-White_White_White Colour Combination. at

- Jugaoge Lencería transparente de encaje para mujer, 1/4 de copas, sin exposición, con aros, brasier, Negro - : Ropa, Zapatos y Joyería

- Ladies Adjustable Strap Cerise Satin Padded Bow Push Up Bras Lingerie 38DD-42DD

- Autre Marque Cadolle Push-Up bra Pink Satin ref.68344 - Joli Closet
