In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

By A Mystery Man Writer
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

Mixture compressibility factor versus temperature at various pressures

Gaseous State

physical chemistry - Pressure vs volume plot for real gas and ideal gas - Chemistry Stack Exchange
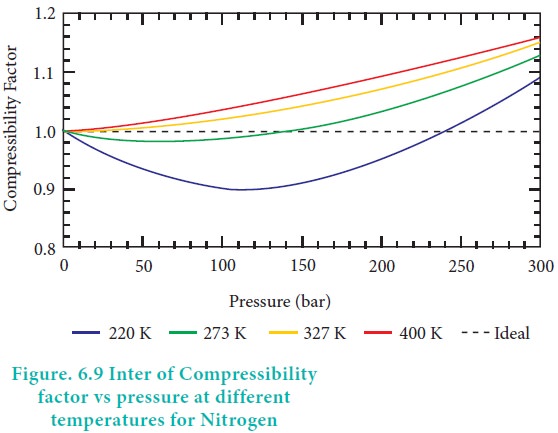
Compressibility factor Z - Gaseous State

47. In the following compressibility factor (Z) vs pressure graph 300 K, the compressibility factor of CH4 pressures < 200 bar deviates from ideal behavior because

Determine Compressibility of Gases

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted as a
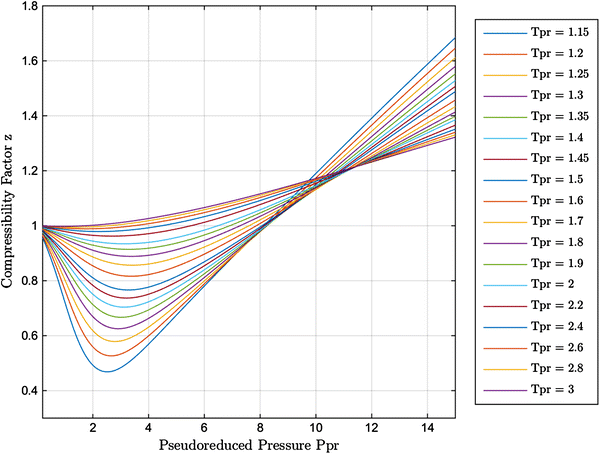
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

College Physical Chemistry] How do I make a Z vs. P plot? : r/HomeworkHelp
What is compressibility factor (Z)? - Sarthaks eConnect
- Math cad compressibility factor, z, of real gas using the redlich

- Gas compressibility factor Z: Ideal gas vs Real gas
- e Compressibility factor (Z) for hydrogen WRT pressure and

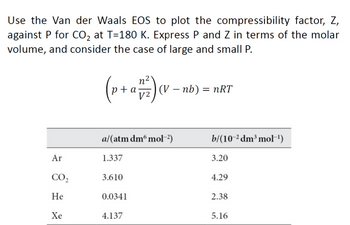
- Answered: Use the Van der Waals EOS to plot the…
- The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n

- Bicicleta Acro / Acro Bike (#110/096) Elder Dragon Brasil - Magic, Pokemon, Yugioh, RPGs e Board Games!

- Bath and Spa Westin Hotel Store

- SHIRT REAL MADRID CAMISETA 2022-2023 VINI JR #20 BRAZIL JERSEY MAILLOT TRIKOT

- Carhartt Women's Rugged Flex Relaxed Fit Canvas Double-Front Pant

- Nike Dri-FIT Game (MLB Atlanta Braves) Men's Long-Sleeve T-Shirt

