The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a

jo 22] What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible.

Compressibility Chart - an overview

16.4: The Law of Corresponding States - Chemistry LibreTexts

Telugu] The compression factor for one mole of real gas at 0^@C and 1
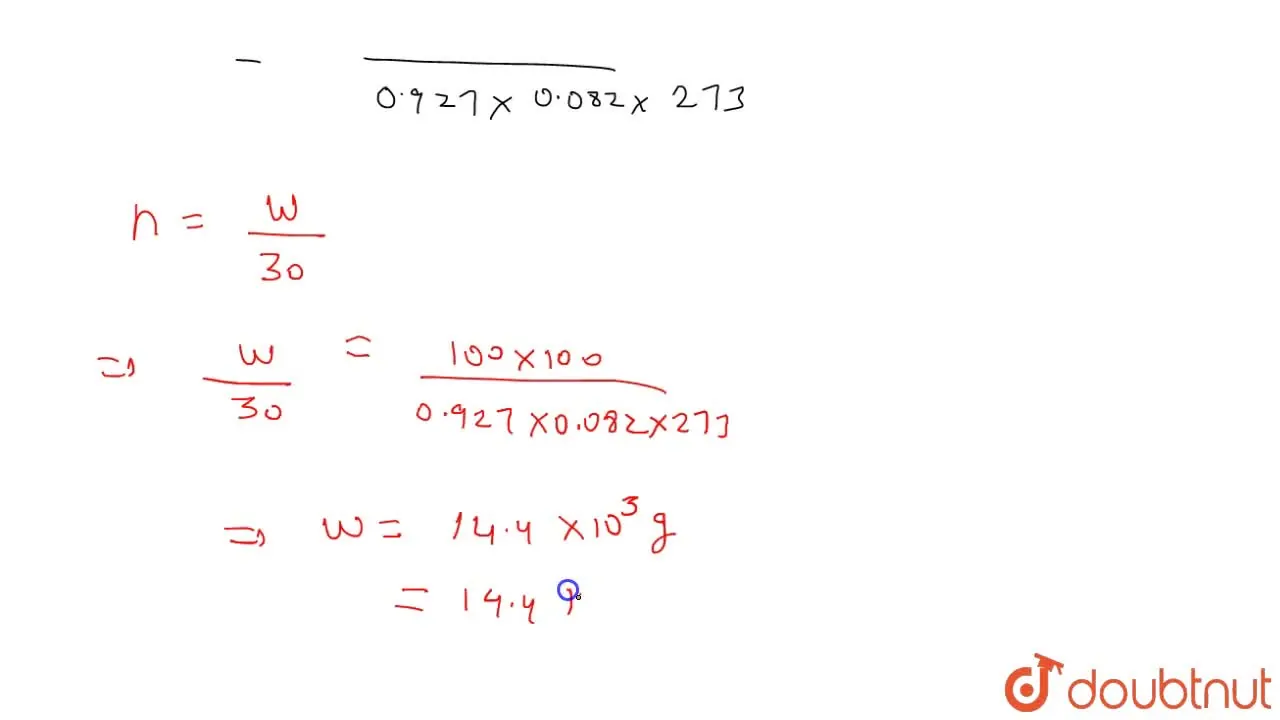
The compressibility factor for a given real gas is 0.927 at 273 K and

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

The compression factor (compressibility factor) for one mole of a v

If `Z` is a compressibility factor, van der Waals' equation at low

Solved Question 1) For water at 293 K and 1 atm, the
- 3.2 Real gas and compressibility factor – Introduction to

- What is the compressibility factor (Z) for 0.02 mole of a van der

- 2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor

- Developing a Thermodynamical Method for Prediction of Activity

- Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

- Hollister Jeans Womens 23 HIgh Rise Jean Legging Advance Stretch Distressed 00

- RBX Womens Embossed Interlock Crop Bra Tank
- EASY BRAID HAIR TEACHING GUIDE TO THREE MAJOR BRAID HAIR STYLES: Practical braid learning guide to many amazing braid styles you can easy learn and

- Nike Solo Swoosh Women's Fleece Pants. Nike.com in 2023

- WDIRARA Women's Graphic Print Elastic Waist Sweatpants Casual Long Joggers, Black, Medium : : Clothing, Shoes & Accessories

