The compression factor (compressibility factor) for 1 mol of a van der

By A Mystery Man Writer
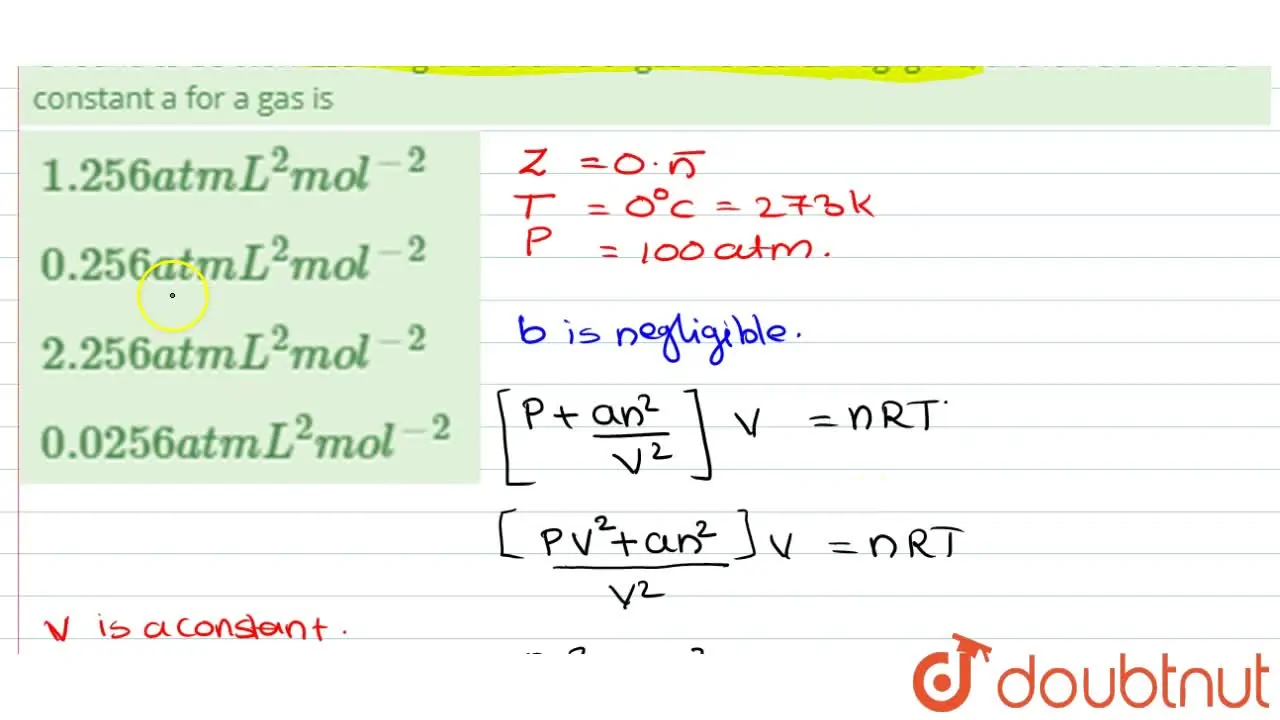
For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)

A 672 ml of a mixture of oxygen - ozone at N.T.P. were found to be wei

Maxwell's speed distribution curve

The ratio of rate of diffusion of gases A and B is 1:4 and their molar

The final product Z in the following reaction is

Two reactants A and B separately shows two chemical reactions.Both rea

The compression factor (compressibility factor) for `1 mol` of a
The compressibility factor of gases is less than unity at STP. Therefo
Malayalam] The compressibility factor for definite amount of van der

A mixture containing 1.12 L of H(2) and 1.12 L of D(2) ( deuterium ) a
For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//
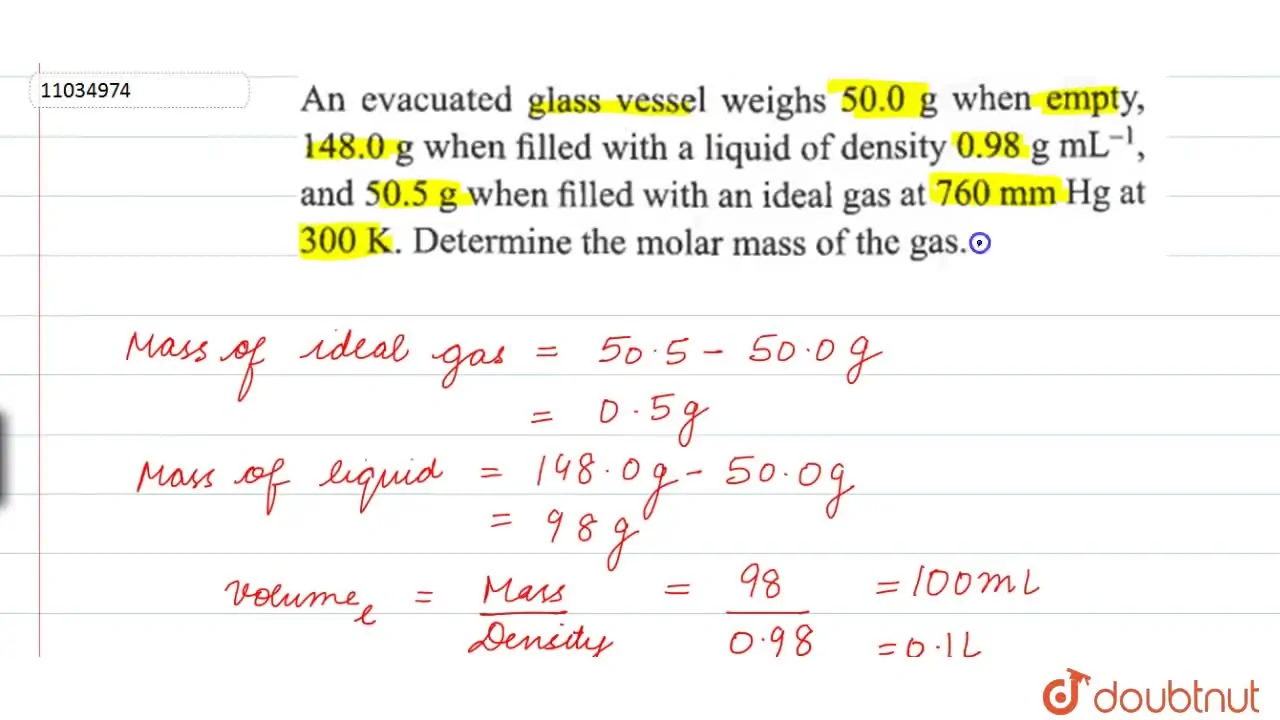
An evacuated glass vessel weighs 50.0 g when empty, 148.0 g when fille

Bengali] What will the value of compressibility factor (Z) be for a g
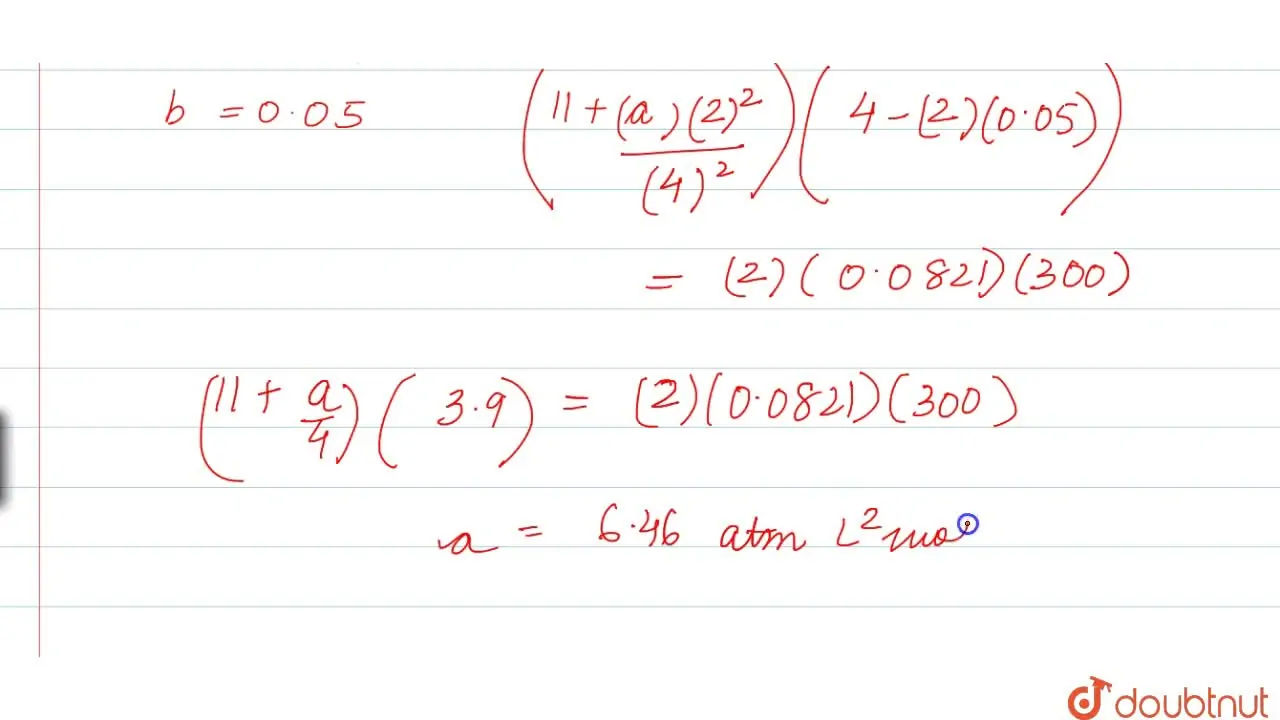
Using van der Waals equation, calculate the constant a when 2 mol of a

The absolute temperature of an ideal gas is….. to/than the average kin
- Solved 1. The compression factor, Z of a gas is 0.625. Which
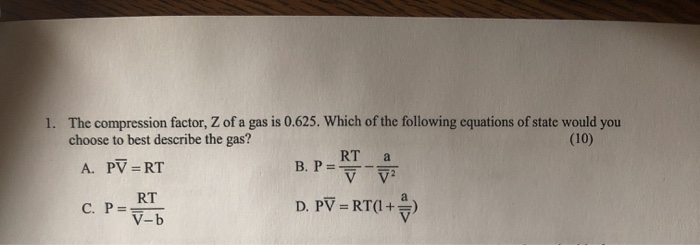
- At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

- Real Gases. The ideal gas equation of state is not sufficient to

- SOLVED: For a gas at a given temperature, the compression factor

- SOLVED: Derive an expression for the compression factor of a gas





